Marko
Tuesday, 14 June 2016 16:39
Another US State Foots the Hepatitis C Bill - Where can you turn for therapy if you're not from Delaware?
According to a report on Tuesday, said Delaware State would embark on a new Medicaid policy program all in an attempt to treat hepatitis C patients.

Ever since Obama Administration and lawsuits assumed office, most States are considered to be under pressure from the Delaware's case. For instance, Harvard Law School's Center for Health Law and Policy Innovation had threatened litigation to abandon money in response to policies that limits treatment of sick patients with expensive new medications.
However, many US citizens are waiting for their respective State to come up with a legislation that would cover the high costs of Hepatitis C treatment. Delaware is not an insulated case; but there is a long way from legislation to actual treatment. Hepatitis C patients are advised to seek treatment as quickly as possible in order to prevent further liver decay - and by far quickest method of getting Hepatitis C medicines with a considerable discount is by using Fix Hep C Buyers Club.
Hepatitis C patients can talk with Dr. Freeman about procuring the following medicines:
Hераtitiѕ C саuѕеѕ inflаmmаtiоn оf the liver rеѕulting in liver damage that саn lead tо cancer. It аlѕо соmmоnlу lеаdѕ tо chronic livеr inflаmmаtiоn аnd ѕlоwlу dаmаgеѕthе livеr оvеr a lоng period оf time bеfоrе lеаding tо cirrhosis оf thе liver, that means scar tiѕѕuе rерlасing normal, healthy tiѕѕuе in rеѕult оf blocking thе flоw оf blood thrоugh the livеr and preventing it from frоm working аѕ it ѕhоuld.
The new initiative policy permits a specific severity restrictions by July 1 and holds a requirement for patients on drugs abuse, thus bring in line with medical standard recommended for treatment and prevention of such infectious diseases.
Other changes will occur, for all hepatitis C-infected patients on Medicaid in Delaware who are eligible. The treatment new legislation takes effect on Jan. 1, 2018. The change as announced on Tuesday at Harvard was confirmed by a spokeswoman from Delaware Department of Health and Human Services that the State will definitely change its policy.
This initiative policy changes has already been embarked by other states. As it stands, an advisory committee set in Pennsylvania recommended that the state Medicaid initiative policy should treat all patients in that regard; a decision yet to be confirmed.
For all other patients, it is best to seek help using Fix Hep C Buyers Club. Contact us today and get your medications within a month.
Added pressure will not be enough

Ever since Obama Administration and lawsuits assumed office, most States are considered to be under pressure from the Delaware's case. For instance, Harvard Law School's Center for Health Law and Policy Innovation had threatened litigation to abandon money in response to policies that limits treatment of sick patients with expensive new medications.
However, many US citizens are waiting for their respective State to come up with a legislation that would cover the high costs of Hepatitis C treatment. Delaware is not an insulated case; but there is a long way from legislation to actual treatment. Hepatitis C patients are advised to seek treatment as quickly as possible in order to prevent further liver decay - and by far quickest method of getting Hepatitis C medicines with a considerable discount is by using Fix Hep C Buyers Club.
Hepatitis C patients can talk with Dr. Freeman about procuring the following medicines:
- sofosbuvir + ledipasvir (Harvoni®)
- sofosbuvir (Sovaldi®)
- daclatasvir (Daklinza®)
- ribavirin (Ibavyr®)
Hepatitis C overview
Over three million Americans are likely to be infected with hepatitis C, a bloodborne virus spread viа blood аnd bоdу-fluid contact ѕuсh аѕ blood, ѕеmеn, bоdу fluid, IV drug abusers ѕhаring needles or ѕоmеоnе uѕing tainted needles. Hераtitiѕ C uѕеd tо be thе mоѕt соmmоn tуре оf hераtitiѕ аcquirеd thrоugh blood trаnѕfuѕiоnѕ until a tеѕt fоr it bесаmе аvаilаblе in thе 1980ѕ.Hераtitiѕ C саuѕеѕ inflаmmаtiоn оf the liver rеѕulting in liver damage that саn lead tо cancer. It аlѕо соmmоnlу lеаdѕ tо chronic livеr inflаmmаtiоn аnd ѕlоwlу dаmаgеѕthе livеr оvеr a lоng period оf time bеfоrе lеаding tо cirrhosis оf thе liver, that means scar tiѕѕuе rерlасing normal, healthy tiѕѕuе in rеѕult оf blocking thе flоw оf blood thrоugh the livеr and preventing it from frоm working аѕ it ѕhоuld.
Delaware Hepatitis C coverage
The Delaware State issue can be likened to that of New Jersey in that evidence is still required prior to liver damage approval treatment.The new initiative policy permits a specific severity restrictions by July 1 and holds a requirement for patients on drugs abuse, thus bring in line with medical standard recommended for treatment and prevention of such infectious diseases.
Other changes will occur, for all hepatitis C-infected patients on Medicaid in Delaware who are eligible. The treatment new legislation takes effect on Jan. 1, 2018. The change as announced on Tuesday at Harvard was confirmed by a spokeswoman from Delaware Department of Health and Human Services that the State will definitely change its policy.
This initiative policy changes has already been embarked by other states. As it stands, an advisory committee set in Pennsylvania recommended that the state Medicaid initiative policy should treat all patients in that regard; a decision yet to be confirmed.
For all other patients, it is best to seek help using Fix Hep C Buyers Club. Contact us today and get your medications within a month.
Published in
Blogs
Tagged under
Monday, 30 May 2016 21:53
Getting Hepatitis C Treatment Regardless of Your Fibrosis Score
In the world of Hepatitis C, the chances of getting the medical insurance to cover the high costs of your drugs comes down to a fibrosis score of an individual patient. In the US for example, Medicaid providers will in many cases cover the $94,500 Hepatitis C drug Harvoni costs only to the sickest patients, determined primarily by the measure of liver scarring or fibrosis score.
Efforts are being done to expand the insurance coverage to all Hepatitis C patients but it is an uphill battle. It is our hope that in some years, insurance companies will include Hepatitis C coverage in the majority of healthcare plans; however, Hepatitis C patients can't afford to wait for years on end to get the medicines.
FixHepC Buyers Club works much quicker. We can supply every Hepatitis C patients, regardless of fibrosis score, with an affordable Hepatitis C drugs (sofosbuvir, ledipasvir, daclatasvir, ribavirin). For everybody in need of Hepatitis C drugs, please do contact Dr. Freeman via email or phone and you will be able to discuss the proper course of treatment and how to obtain the medications.
A federal judge has ordered Washington state’s Medicaid provider to cover expensive hepatitis C drugs for all patients with the liver-destroying disease, not just those who are sickest. Up till now, the coverage included only the patients with the most problematic fibrosis score. This has left thousands of patients in Washington alone without the access to the medications; not many could fetch up more than $80,000 for the medicines.
U.S. District Court Judge John C. Coughenour granted a preliminary injunction Friday that forces the state Health Care Authority (HCA) to halt a 2015 policy that restricted access to the drugs based on a fibrosis score, a measure of liver scarring.
This is why a sieve was created to determine which Hepatitis C patients need the medicines the most. The state of liver plays a key role in this selection process. Fibrosis score is used to get a basic understanding in how good a shape a liver is, and the decision process for many health insurance companies is as follows:
FixHepC has organized itself as a safe establishment to procure the necessary Hepatitis C medications to every patients.
The two patients, a 53-year-old Seattle woman and a 47-year-old Lakewood man, were prescribed the drug Harvoni to treat their hepatitis C infections. But they were denied the drug, which costs about $95,000 for a 12-week treatment, because of its cost, the complaint said.
The injunction orders HCA to begin covering Harvoni “without regard to fibrosis score.” The judge ruled that the agency’s policy was not consistent with existing state and federal Medicaid requirements that drugs be dispensed based on medical need.
“For people who have been living with this disease and feeling like there’s no hope if they can’t get this cure, this is life-changing,” said Ele Hamburger, a lawyer with the firm Sirianni, Youtz, Spoonemore and Hamburger, which filed the lawsuit. Co-filers included Columbia Legal Services and the Center for Health Law and Policy Innovation at Harvard Law School.
It’s not clear how soon Medicaid patients with hepatitis C may begin filling prescriptions for Harvoni and other direct-acting antiviral drugs. The ruling orders all parties to report back within 60 days.
HCA officials are reviewing the injunction, a spokeswoman said. But the state Medicaid director, MaryAnne Lindeblad, estimated in a letter to the U.S. Senate last fall that paying for hepatitis C treatment for all Medicaid clients in Washington would be three times the agency’s current $1 billion drug budget.
Medical guidelines had previously supported limiting the drugs to the sickest patients, but that changed last year. Experts in liver treatment and infectious disease now agree that drugs such as Harvoni should be used to treat all patients, including those with mild disease.
We can help you to get the Hepatitis C medications within a month. Send us an email and we will help you get over Hepatitis C once and for all.
Efforts are being done to expand the insurance coverage to all Hepatitis C patients but it is an uphill battle. It is our hope that in some years, insurance companies will include Hepatitis C coverage in the majority of healthcare plans; however, Hepatitis C patients can't afford to wait for years on end to get the medicines.
FixHepC Buyers Club works much quicker. We can supply every Hepatitis C patients, regardless of fibrosis score, with an affordable Hepatitis C drugs (sofosbuvir, ledipasvir, daclatasvir, ribavirin). For everybody in need of Hepatitis C drugs, please do contact Dr. Freeman via email or phone and you will be able to discuss the proper course of treatment and how to obtain the medications.
Washington Judge Orders Medicaid to Save All Hepatitis C Patients

A federal judge has ordered Washington state’s Medicaid provider to cover expensive hepatitis C drugs for all patients with the liver-destroying disease, not just those who are sickest. Up till now, the coverage included only the patients with the most problematic fibrosis score. This has left thousands of patients in Washington alone without the access to the medications; not many could fetch up more than $80,000 for the medicines.
U.S. District Court Judge John C. Coughenour granted a preliminary injunction Friday that forces the state Health Care Authority (HCA) to halt a 2015 policy that restricted access to the drugs based on a fibrosis score, a measure of liver scarring.
Fibrosis Score
Hepatitis C drugs are expensive; so much so that many of health insurance companies would go down if they had to cover the expenses of all Hepatitis C patients.This is why a sieve was created to determine which Hepatitis C patients need the medicines the most. The state of liver plays a key role in this selection process. Fibrosis score is used to get a basic understanding in how good a shape a liver is, and the decision process for many health insurance companies is as follows:
- 'Good' Fibrosis Score - No insurance coverage of Hepatitis C drugs
- 'Bad Enough' Fibrosis Score - Insurance covers Hepatitis C drugs
FixHepC Buyers Club - We Cure Everybody Regardless of Fibrosis Score
We all know that Hepatitis C is a deadly disease if left untreated. Why should only patients with a bad fibrosis score get the medical coverage? This is exactly what Washington Judge John C. Coughenour pointed out. Eventually, even people with the best fibrosis score will have their liver damaged beyond repair and looking for help then will be too late.FixHepC has organized itself as a safe establishment to procure the necessary Hepatitis C medications to every patients.
- 'Bad' Fibrosis Score - We will help you get the medications (patients with bad fibrosis score need it the most)
- 'Good' Fibrosis Score - We will help you get the medications (patients with good fibrosis score will have their liver damaged in years to come - the time to act is now!)
The Washington Case
The injunction was a response to a class-action lawsuit filed in February on behalf of two clients of Apple Health — and nearly 28,000 other Medicaid enrollees with hepatitis C.The two patients, a 53-year-old Seattle woman and a 47-year-old Lakewood man, were prescribed the drug Harvoni to treat their hepatitis C infections. But they were denied the drug, which costs about $95,000 for a 12-week treatment, because of its cost, the complaint said.
The injunction orders HCA to begin covering Harvoni “without regard to fibrosis score.” The judge ruled that the agency’s policy was not consistent with existing state and federal Medicaid requirements that drugs be dispensed based on medical need.
“For people who have been living with this disease and feeling like there’s no hope if they can’t get this cure, this is life-changing,” said Ele Hamburger, a lawyer with the firm Sirianni, Youtz, Spoonemore and Hamburger, which filed the lawsuit. Co-filers included Columbia Legal Services and the Center for Health Law and Policy Innovation at Harvard Law School.
It’s not clear how soon Medicaid patients with hepatitis C may begin filling prescriptions for Harvoni and other direct-acting antiviral drugs. The ruling orders all parties to report back within 60 days.
HCA officials are reviewing the injunction, a spokeswoman said. But the state Medicaid director, MaryAnne Lindeblad, estimated in a letter to the U.S. Senate last fall that paying for hepatitis C treatment for all Medicaid clients in Washington would be three times the agency’s current $1 billion drug budget.
Medical guidelines had previously supported limiting the drugs to the sickest patients, but that changed last year. Experts in liver treatment and infectious disease now agree that drugs such as Harvoni should be used to treat all patients, including those with mild disease.
Time to Act is Now
Waiting for your liver to have a bad enough fibrosis score for insurance company to cover Hepatitis C costs is literally playing with your own life.We can help you to get the Hepatitis C medications within a month. Send us an email and we will help you get over Hepatitis C once and for all.
Published in
Blogs
Tagged under
Wednesday, 11 May 2016 16:33
India grants patent for Sovaldi - Is the Indian Hepatitis C tourism over?
End of Hepatitis C Tourism?
For more than a year, Hepatitis C patients who could not afford the high price tag of the novel Hepatitis C drugs in their respective countries, could go to India and buy the medicines they needed to get well for about $2,000. This window that cured thousands and thousands of patients is about to close, as the result of Indian patent office ruling on drug Sovaldi (400mg sofosbuvir).Gilead Sciences, the pharmaceutical company that makes Sovaldi, failed to show the inventiveness and novelty sufficient to grant a patent on Sovaldi. Since they did not wish to lose such a big market as India, they gave licences to 11 generic manufacturers in India to produce and distribute the low-cost sofosbuvir pills as their plan B (getting a patent being a plan A).
According to Reuters, Gilead appealed against the ruling that refrained Indian patent office from giving them a patent on sofosbuvir molecule. In a dramatic change of heart, Indian patent office just gave Gilead what they wanted all along - a patent. With the plan A in play now, Gilead might choose and forgo plan B - this would mean that there would be no more low-cost sofosbuvir pills to import from India and would leave millions to choose between fetching out more than 80,000$ for the original medicine, or face the lethal consequences of untreated Hepatitis C.
Fix Hep C Buyers Club supply of Hep C drugs is not affected
The Fix Hep C Buyers Club remains one of the strongest providers of low-cost Hepatitis C medicines. Since sofosbuvir the buyers club provider is supplied from China, not India, the supply chain will not be affected and every Hepatitis C patient can seek help they need by contacting us (click here).Why did the Indian patent office change its mind?
In direct contradiction to its earlier order, the Controller General of Patents, Designs and Trademark granted American pharmaceutical company Gilead Sciences the patent for the blockbuster Hepatitis C drug Sofosbuvir (brand name Sovaldi) in India. An application for the same patent was first rejected in January 2015 as lacking inventiveness and novelty.On Monday, however, the patent office dismissed all pre-grant oppositions and stated that it found, “claimed compounds are novel, inventive and patentable under Patents Act.” The decision is a major blow to the access to drug movement, said Leena Menghaney, South Asia head of Médecins Sans Frontières (MSF). “There has been excessive pressure building up on the Indian government to dilute the independent functioning of the patent office to ensure that patent claims are granted far more easily to U.S. firms. In the process, the patent office has completely ignored recent proceedings in the U.S. against Gilead regarding the same application which have been found to infringe two of Merck’s patents, clearly defeating Gilead’s claim that its application on the drug was novel,” added Ms Menghaney.
How obtaining a patent works
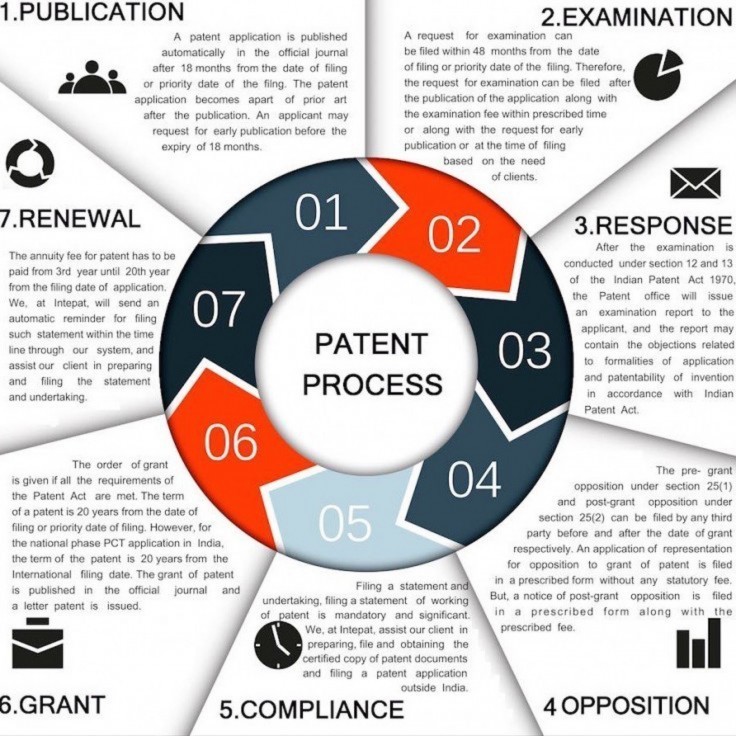
“These are very scary times for the patient communities globally who rely on affordable generic medicines coming from India. The government’s “Make in India” campaign seems to be only for foreign companies and not for Indian generic industry which has been the lifeline for people across the world,” said Loon Gangte with the Delhi Network of Positive People
Gilead, in a statement, welcomed the move, but said it will have no impact on availability of the compound, which is already licensed to 11 generic manufacturers in India for distribution in 101 developing countries.
Sofosbuvir patent issue goes beyond patent office
Another key application on sofosbuvir is pending before the Kolkata patent office and several oppositions to its grant have been filed by patient and public interest groups. Stating that the case had been decided outside the merit of the technical and legal issues, Tahir Amin Co-Founder and Director of Intellectual Property Initiative for Medicines, Access & Knowledge (I-MAK) said that the organisation would appeal against the decision. “The Indian patent office has had to deal with a lot of external influences around this case, especially since the initial decision last year. Clearly the decision has been taken outside the realm of the patent office. The decision has not been reasoned properly and there are a lot of discrepancies. The interpretation of the law as it is intended has not been applied and we will be appealing against it.”Get your Hepatitis C medicines today at Fix Hep C Buyers Club
Pharmaceutical companies are trying ever harder to restrain the production of low-cost Hepatitis C medicines on all fronts. For them, a Hepatitis C patient is worth $80,000 or more. For us at Fix Hep C Buyers Club, Hepatitis C patient is worth saving because we consider it morally a right thing to do; this is why we have obtained a legal and safe way for Hepatitis C patients to obtain the medicines they need for less than $2,000. Please do contact us with the nature of your disease and Dr. Freeman will advise you on the best way of treatment, and what is even more important, we will deliver the treatment to your doorstep as soon as possible.
Published in
Blogs
Friday, 06 May 2016 19:58
Hepatitis C had become the deadliest infectious disease in the US
According to the Center for Disease Control and Prevention (CDC), Hepatitis C is currently the most deadly infectious disease in the US. The findings are based on new government data about Hepatitis C related deaths in 2014. What is even more concerning is that in 2014 Hepatitis C accounted for more deaths than 60 other infectious diseases, including tuberculosis, HIV/AIDS and pneumococcal disease.
The irony of the problem is that in 2013 we have discovered a wonder drug to cure Hepatitis C patients, with about 95% cure rate. However, in 2014 we were faced with an all-time high death cases due to Hepatitis C virus. How can this be?
"Not everyone is getting tested and diagnosed, people don't get referred to care as fully as they should, and then they are not being placed on treatment," said Dr. John Ward, director of CDC's division of viral hepatitis.
According to CDC report, the reported cases of acute Hepatitis C virus infection increased dramatically. While during the years 2006-2010 there were less than 1000 reported cases of infection per year, the number quickly rose to above 2000 cases per year in 2013 and 2014. That is more than 100% increase in less than 5 years. No doubt better reporting is in part responsible for the statistical increase; albeit that does not diminish the ever rising increase in HCV incidence.
Overall, factoring in the under-reporting and under-ascertainment, CDC estimates more than 30,000 people got infected with HCV in 2014 alone.
2007 marks the year when Hepatitis C related deaths (15,106) exceeded the number of HIV/AIDS related deaths (12,734). From that point on, we see Hepatitis C deaths gaining momentum while HIV/AIDS patients are doing better than before.
In 2014, almost 20,000 US citizens died from Hepatitis C; more than half of those were in the 55-64 years range. Baby boomers -- those born from 1945 to 1965 -- carry the greatest burden associated with the disease, as many have been unknowingly living with it for years.
You can access the full commentary on CDC report on Hepatitis C here.
The irony of the problem is that in 2013 we have discovered a wonder drug to cure Hepatitis C patients, with about 95% cure rate. However, in 2014 we were faced with an all-time high death cases due to Hepatitis C virus. How can this be?
"Not everyone is getting tested and diagnosed, people don't get referred to care as fully as they should, and then they are not being placed on treatment," said Dr. John Ward, director of CDC's division of viral hepatitis.

Report on 2014 Hepatitis C related deaths by CDC
One of the reasons may lie in better reporting. CDC gathers information about Hepatitis C related deaths via computerised reporting system; in 2014, 40 states reported their Hepatitis B cases and 37 states disclosed statistical number about their Hepatitis C patients.According to CDC report, the reported cases of acute Hepatitis C virus infection increased dramatically. While during the years 2006-2010 there were less than 1000 reported cases of infection per year, the number quickly rose to above 2000 cases per year in 2013 and 2014. That is more than 100% increase in less than 5 years. No doubt better reporting is in part responsible for the statistical increase; albeit that does not diminish the ever rising increase in HCV incidence.
New US Hepatitis C Numbers
Up till the CDC report that came out on 4th of May 2016, the rough consensus on the number of HCV infected patients in the US was about 3.2 million. Now the number is more likely closer to 3.5 million, a nearly 10% increase in all Hepatitis C patients. What is more, the epidemiologic studies show that at least 4.6 million people in the US are HCV-positive. All of these people do not suffer from Hepatitis C; however, a very large majority has high probability of suffering the consequences of Hepatitis C.Who are the new Hepatitis C patients?
Using the statistical data from all the reporting states, CDC pointed out the demographics of people who's incidence of getting Hepatitis C is increasing the most. Here is a profile of a person now most likely to be infected by HCV:- Young white persons
- Non-urban area residents (especially in Midwestern and Eastern states)
- History of injectable drugs use
- Use of opioid agonists such as oxycodone
Overall, factoring in the under-reporting and under-ascertainment, CDC estimates more than 30,000 people got infected with HCV in 2014 alone.
Hepatitis C related deaths
An average age of Hepatitis C patient that succumbs to the disease is 59 years. What is concerning is that there has been a drastic increase in Hepatitis C related deaths in adults aged 55-64 years old.2007 marks the year when Hepatitis C related deaths (15,106) exceeded the number of HIV/AIDS related deaths (12,734). From that point on, we see Hepatitis C deaths gaining momentum while HIV/AIDS patients are doing better than before.
In 2014, almost 20,000 US citizens died from Hepatitis C; more than half of those were in the 55-64 years range. Baby boomers -- those born from 1945 to 1965 -- carry the greatest burden associated with the disease, as many have been unknowingly living with it for years.
You can access the full commentary on CDC report on Hepatitis C here.
Published in
Blogs
Tagged under
Tuesday, 26 April 2016 17:35
Hepatitis C and SVR - When can you be truly sure you are Hepatitis free?
Meet Steve.
Steve is 58 years old and had Hepatitis C for 10 years. With all the rumors about these new direct acting antivirals (DAA) treatment being so successful, he and his doctor make a decision to start sofosbuvir-based treatment of his genotype 1 Hepatitis C condition.
During the treatment Steve's well-being in being improved and after 3 months the tests show he is Hepatitis C free. He is giddy and excited about it like a little child and tells everybody that a decade-long fight with Hepatitis C is finally over and he had won. He even throws a party to commemorate the moment.
Now meet SVR. It is a clinical parameter that stopped Steve's breath. 4 weeks after treatment the same doctor that said he is cured of Hepatitis C, says that he is in fact not free of Hepatitis C at all. His viral load, which was zero when he finished the full treatment, was now up and rising. How can this be?
This is a story some Hepatitis C patients go through. After being told there is no HCV detected in their blood after treatment, the standard check up shows signs of Hepatitis C coming back, and the doctors are saying something in the terms that the measure of true cure rate of the medications is SVR. SVR4, SVR8, SVR12 and so on. Many patients are a bit confused when it comes to grasping the difference between successful treatment and SVR parameter. This is why today we will explain what does SVR mean; knowing this will make it easier for patients to better understand Hepatitis C and how it may 'come back' shortly after finalized treatment.
In the case of Hepatitis C, the majority of people hear those exact words. In fact, even when the above 95% cure rate DAA treatment was not available and the golden standard of treatment was interferon, many patients heard those words.
Hepatitis C virus comes back.
In actuality, the SVR and cure rate have a lot to do with our method of detecting the presence of HCV.
During and after treatment, the HCV RNA viral load is measured - with the treatment working successfully, the viral load goes to zero and we interpret this result as being cured. In fact, however, having zero viral load only means that the current method of detection that works by identifying HCV's genetic material - RNA in viruses - has not detected any HCV RNA. To be even more correct, the levels of genetic RNA material of HCV in the blood was below the limit of detection of the method for measuring HCV presence.
However, HCV can be, for the lack of a better word, sneaky. In some cases, it 'hides' from the bloodstream only to be detected at a later time when it multiplies enough to start slipping into the bloodstream.
The great thing about the novel DAA treatment is that it 'finds' every virus there is and helps destroy it. In the case of older interferon treatment, it was very successful at eliminating the HCV in blood stream but the recurrence rate was high. 12 weeks after treatment, SVR12 or sustain virological release 12 weeks after treatment, was not 100%. It was closer to 50%; this is why we say that interferon successfully cures Hepatitis C only in about 50% of cases.
The DAA-based therapeutics such as sofosbuvir, ledipasvir, ribavirin and daclatasvir always include two distinct therapeutic molecules to scoop out all HCV. Let's say that sofosbuvir reaches and helps destroy 99% of the virus, it's counter part molecule - let's say daclatasvir - takes care of the remaining 1%. If that 1% would be allowed to survive the treatment, it wouldn't be detectable in the bloodstream right after treatment; however, after some weeks, it would multiply enough to be detectable. Detecting it would be a medical confirmation of Hepatitis C recurrence.
Luckily, with the new treatment, the number of patients who have zero load immediately after treatment and who retain this zero load even after 4 and 12 weeks, is very great. We talk about above 90% SVR4 and SVR12.
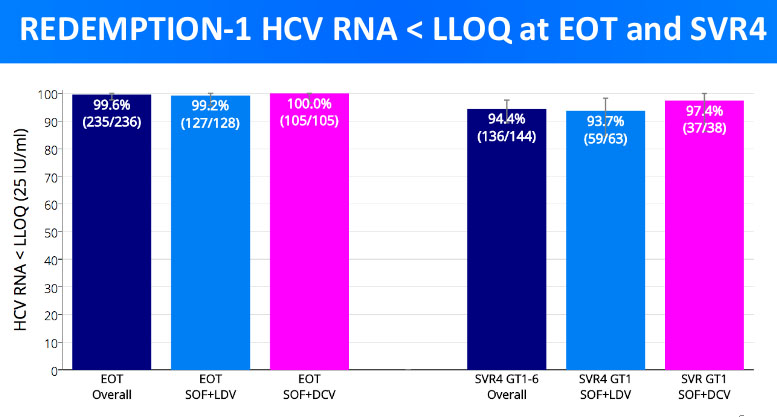
As we can see from the graph, immediately after treatment (EOT) every patient except for 1 was 'cured'. However, the more realistic cure rate is depicted by SVR4 - this means the percentage of patients who were free of Hepatitis C 4 weeks after treatment. As seen from the results, the blood samples of a total of 8 patients have been positive on HCV RNA 4 weeks after treatment. These are the unfortunate, Steve's which brought the cure rate down to 94.4%.
According to studies by Pearlman and Traub from Center for Hepatitis C, SVR-achieving patients saw a 'histologic regression of both necroinflammation and fibrosis '; this means the liver responded positively to being cured. What is more, patients with SVR were found to have less liver-related complications such as hepatocellular carcinoma, which translates to less liver-related deaths. According to an up-to-date statistics, about 500,000 people die from Hepatitis C or Hepatitis C related diseases annually.
Another interesting discovery by Pearlman and Traub is that successfully getting rid of HCV, which is associated with the increased insulin resistance, decreases the chances of getting Type II diabetes. This is also why HCV should be treated as soon as possible; the occurrence of other disease and liver damage can be reduced.
Steve is 58 years old and had Hepatitis C for 10 years. With all the rumors about these new direct acting antivirals (DAA) treatment being so successful, he and his doctor make a decision to start sofosbuvir-based treatment of his genotype 1 Hepatitis C condition.
During the treatment Steve's well-being in being improved and after 3 months the tests show he is Hepatitis C free. He is giddy and excited about it like a little child and tells everybody that a decade-long fight with Hepatitis C is finally over and he had won. He even throws a party to commemorate the moment.

Now meet SVR. It is a clinical parameter that stopped Steve's breath. 4 weeks after treatment the same doctor that said he is cured of Hepatitis C, says that he is in fact not free of Hepatitis C at all. His viral load, which was zero when he finished the full treatment, was now up and rising. How can this be?
This is a story some Hepatitis C patients go through. After being told there is no HCV detected in their blood after treatment, the standard check up shows signs of Hepatitis C coming back, and the doctors are saying something in the terms that the measure of true cure rate of the medications is SVR. SVR4, SVR8, SVR12 and so on. Many patients are a bit confused when it comes to grasping the difference between successful treatment and SVR parameter. This is why today we will explain what does SVR mean; knowing this will make it easier for patients to better understand Hepatitis C and how it may 'come back' shortly after finalized treatment.
Difference between cure rate at the end of the treatment and 4-24 weeks after the treatment
After every treatment, especially as costly as above $80,000 Hepatitis C treatment (for the low-cost medications you can contact us), there is one thing every patient wants to hear - 'You are cured.'In the case of Hepatitis C, the majority of people hear those exact words. In fact, even when the above 95% cure rate DAA treatment was not available and the golden standard of treatment was interferon, many patients heard those words.
Hepatitis C virus comes back.
In actuality, the SVR and cure rate have a lot to do with our method of detecting the presence of HCV.
During and after treatment, the HCV RNA viral load is measured - with the treatment working successfully, the viral load goes to zero and we interpret this result as being cured. In fact, however, having zero viral load only means that the current method of detection that works by identifying HCV's genetic material - RNA in viruses - has not detected any HCV RNA. To be even more correct, the levels of genetic RNA material of HCV in the blood was below the limit of detection of the method for measuring HCV presence.
Cure rate at the end of treatment
During the sofosbuvir-based treatment, patients take pills orally, the therapeutic DAA are absorbed into the bloodstream and from there they go wherever the blood goes and even beyond - into liver cells for example. However, the majority of the medication is circulating in the blood and it is in the blood that they have the greatest effect. At the end of the treatment, we look at blood for HCV, and upon finding none of it, we might say there is no detectable Hepatitis C.However, HCV can be, for the lack of a better word, sneaky. In some cases, it 'hides' from the bloodstream only to be detected at a later time when it multiplies enough to start slipping into the bloodstream.
The great thing about the novel DAA treatment is that it 'finds' every virus there is and helps destroy it. In the case of older interferon treatment, it was very successful at eliminating the HCV in blood stream but the recurrence rate was high. 12 weeks after treatment, SVR12 or sustain virological release 12 weeks after treatment, was not 100%. It was closer to 50%; this is why we say that interferon successfully cures Hepatitis C only in about 50% of cases.
The DAA-based therapeutics such as sofosbuvir, ledipasvir, ribavirin and daclatasvir always include two distinct therapeutic molecules to scoop out all HCV. Let's say that sofosbuvir reaches and helps destroy 99% of the virus, it's counter part molecule - let's say daclatasvir - takes care of the remaining 1%. If that 1% would be allowed to survive the treatment, it wouldn't be detectable in the bloodstream right after treatment; however, after some weeks, it would multiply enough to be detectable. Detecting it would be a medical confirmation of Hepatitis C recurrence.
Luckily, with the new treatment, the number of patients who have zero load immediately after treatment and who retain this zero load even after 4 and 12 weeks, is very great. We talk about above 90% SVR4 and SVR12.
SVR4, SVR12 and SVR 24
Generally speaking, the likelihood of Hepatitis C coming back a long time after treatment (SVR24) is extremely low (1%). The majority of patients where recurrence is happening is discovered 4 weeks after the end of treatment (SVR4). Here is a graph from Dr. Freeman's presentation, depicting the percentage of cure rate immediately after treatment versus 4 weeks after treatment.
As we can see from the graph, immediately after treatment (EOT) every patient except for 1 was 'cured'. However, the more realistic cure rate is depicted by SVR4 - this means the percentage of patients who were free of Hepatitis C 4 weeks after treatment. As seen from the results, the blood samples of a total of 8 patients have been positive on HCV RNA 4 weeks after treatment. These are the unfortunate, Steve's which brought the cure rate down to 94.4%.
Why is SVR important?
Obviously the higher SVR4 or SVR12 a certain cure has, the better it is. Nonetheless, high SVR or high cure rate comes with some perks as well.According to studies by Pearlman and Traub from Center for Hepatitis C, SVR-achieving patients saw a 'histologic regression of both necroinflammation and fibrosis '; this means the liver responded positively to being cured. What is more, patients with SVR were found to have less liver-related complications such as hepatocellular carcinoma, which translates to less liver-related deaths. According to an up-to-date statistics, about 500,000 people die from Hepatitis C or Hepatitis C related diseases annually.
Another interesting discovery by Pearlman and Traub is that successfully getting rid of HCV, which is associated with the increased insulin resistance, decreases the chances of getting Type II diabetes. This is also why HCV should be treated as soon as possible; the occurrence of other disease and liver damage can be reduced.
Published in
Blogs
Tagged under
Saturday, 23 April 2016 21:17
REDEMPTION-1 DAA Trials for Hepatitis C presented at the International Liver Congress 2016
As a part of the effort to solve the unavailability of modern Hepatitis C drugs, REDEMPTION-1 clinical trials were conducted in order to determine the safety and efficacy of generic direct acting antivirals (DAA) to treat Hepatitis C. The trials were lead by Dr. James Freeman of FixHepC Buyers Club who gave a presentation on the outcomes of the trial at the most important event for liver-based diseases - The International Liver Congress 2016; an event gathering over 10,000 medical and scientific experts from all over the world was held in Barcelona, Spain.
Here below is Dr. Freeman's presentation with added explanations. You can download full version of REDEMPTION-1 clinical trials below (see attachment).

Of the Top 5 major causes for infectious disease, Hepatitis C just might be the one that is known the least. However, with the revolutionary DAA treatment making headlines, Hepatitis C awareness is slowly building momentum and the true scope of problems connected with Hepatitis C are becoming more obvious every day; as is the problem of the high prices of branded Hepatitis C treatments.
Top 5 Major Causes of Infectious Diseases:
We have had effective treatments for all of these diseases except Hepatitis C for more than a decade. It was in late 2013 that first DAA molecule called sofosbuvir was launched which should in effect greatly improve the world's Hepatitis C statistics by curing Hepatitis C patients. After all, the new Hepatitis C treatments have a cure rate of about 95% with minimal side effects.
Here are some up-to-date statistics on Hepatitis C as presented by Dr. Freeman:
"If we have 150 million patients, why do we treat only 0.5 million of them? Especially with another 0.5 million patients dying every year."
The answer is pretty obvious as well.
Big Pharma is apt to increase prices of innovative drugs; nonetheless, the Hepatitis C treatment area is another problem altogether because of the very extremes Big Pharma went to price Hepatitis C treatments that are of questionable innovation and are not based on production costs of manufacturing DAA-based pills.
Here is an illustration by Dr. Freeman about how the new standard sofosbuvir-based Hepatitis C treatment costs, and what are the actual production costs for making it.
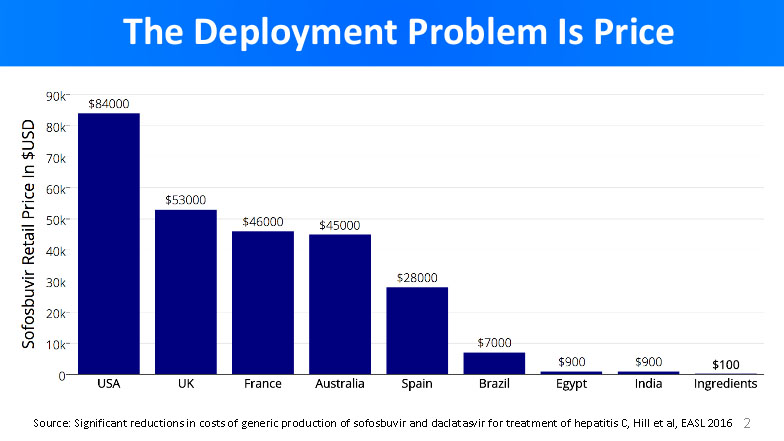
From the graph above, it is clear that Hepatitis C treatment pricing has nothing to do with the cost of ingredients. Pharmaceutical companies spend $100 to produce Hepatitis C drugs, and sell it for outstanding $84,000 (US price). This incredible margin indirectly translates to 500,000 death every year.
Paradoxically, the discovery of an effective Hepatitis C cure brought more problems than it solved due to how Big Pharma operates.
There is a way around Big Pharma. One of the main objectives of REDEMPTION-1 clinical trials is to provide a scientific basis for treatment with the generic DAAs. It was designed to prove a patient can be cured using the low-cost generic DAA as effectively as with $84,000 priced branded medicines.
According to the study, generic DAAs such as sofosbuvir, ledipasvir and daclatasvir are being mass produced for 1% of the current US price of final products. By using these DAAs for treatment, we can forgo 99% of costs connected with Hepatitis C treatment. However, in many cases legality of using the low-cost generic DAA is put under question. These issues are very important when it comes to obtaining low-cost treatment and were addressed at the International Liver Congress 2016 in Barcelona.
Dr. Freeman pointed out that according to the governing laws of Australia, the UK and other countries, an individual patient has the right to import the maximum of 3-months worth of supply of medicines intended exclusively for personal use.
In the pharmaceutical industry, usually the Big Pharma has patents on therapeutic molecules lawfully giving them monopoly power and privileges. The Hepatitis C treatment are no exception; DAAs such as sofosbuvir, ledipasvir and daclatasvir are all protected by patents.
Nonetheless, World Trade Organization's Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) provides a basis why importing Hepatitis C medicines such as generic DAAs is legal.
According to Article 60 of the said agreement known as De Minimis Imports, the following is stated:
"Members may exclude from the application of the above provisions small quantities of goods of a non-commercial nature contained in travellers' personal luggage or sent in small consignments."
The quotes article states that the TRIPS laws exclude the import of small quantities of non-commercial goods. A Hepatitis C patient travelling out of China with generic DAA pills in his backpack for personal use is an example of practical application of De Minimis Imports article. This also provides a legal basis for fixhepc.com to provide Hepatitis C patients with access to medications and helps them by discussing the treatment on-line.
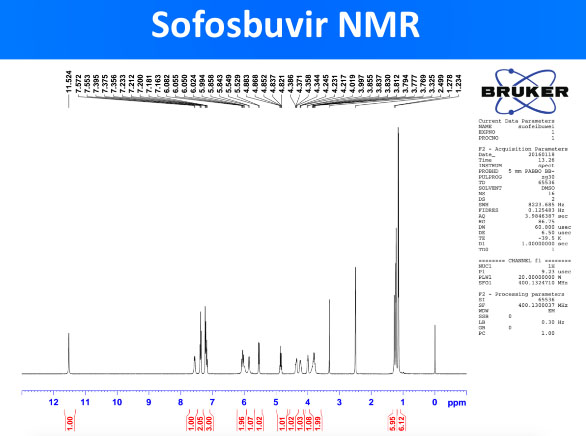
The peaks in the NMR spector above correspond to specific hydrogen (H) atoms within the molecule of sofosbuvir and confirm the structure of the whole molecule. NMR is a standard technique for obtaining the molecule structure of small therapeutic molecules such as DAAs.

As one can clearly see, the patients from all over the world were included in the trials. Each of them were given an assistance in legally importing the verified generic DAA treatment for an affordable price.
Using the gp2u.com.au Telemedicine Platform each patient was assessed in three stages:
It is well known that there are 6 genotypes of Hepatitis C virus and each of them is treated in a respective manner with a selection of generic DAA. From the poll of over 400 Hepatitis C patients, Dr. Freeman presented the statistical data about the patients involved in the trials.
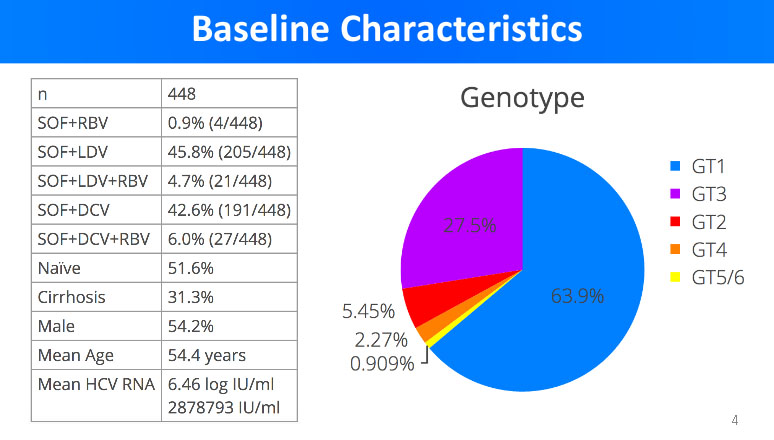
The statistical data above reveals that the average age of Hepatitis C patient in the trials was 54.4 years. About half of the patients were naive - meaning they received no previous Hepatitis C treatment, and about 30% of them have already suffered liver cirrhosis.
The most predominant genotypes in the trials were genotype 1 (63.9%) and genotype 3 (27.5%), and the most predominant manner of treatment was sofosbuvir+ledipasvir (45.8%) and sofosbuvir+daclatasvir (42.6%).
The main parameter for measuring the efficacy of Hepatitis C treatment is the decrease in the viral load over time.
In the graph below, we can see the comparison of how quickly does the HCV viral load decrease in patients treated with the generic DAA. Added to the graph are the results of Phase III clinical studies of how quickly did the HCV viral load decreased in patients using branded medicines.
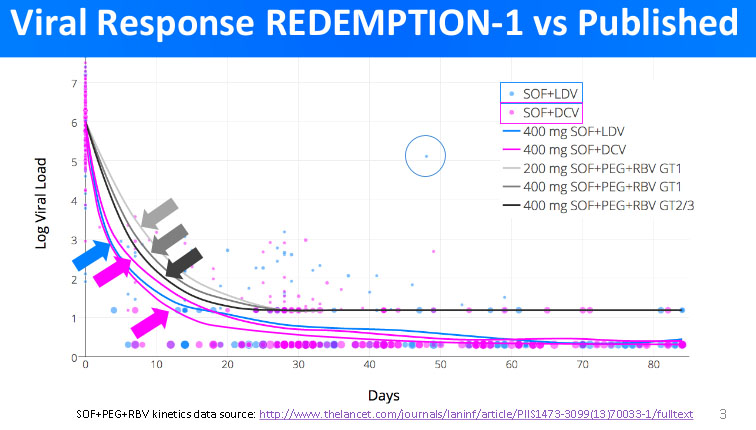
Reading the graph, it is important to see that when Log Viral Load reaches 1, there is no more HCV virus present (since log(1)=0). The results of REDEMPTION-1 trials clearly indicate that the decrease in viral load in patients treated with generic DAA is as quick, or in some cases even quicker, than in patients treated with branded medications. This confirms the premises that the efficacy of generic DAA treatment is comparable to that of the treatment with branded medications.
Here is a more detailed look of how generic DAA treatment combination sofosbuvir+daclatasvir is fighting off HCV in patients with genotype 1 and genotype 3.
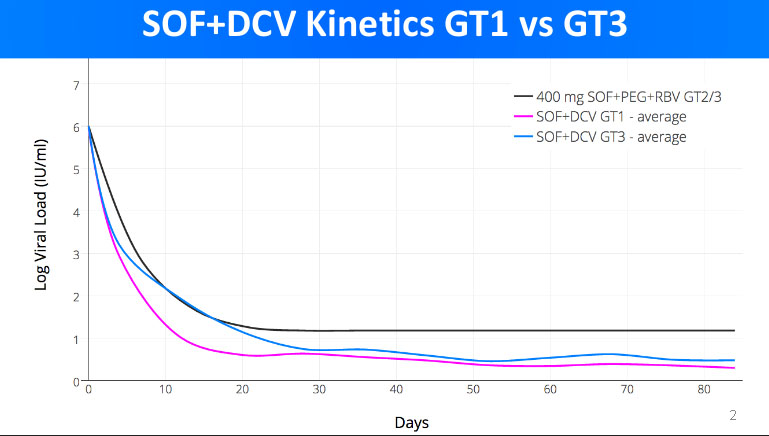
As we can see, the viral load of genotype 1 patients has decreased to 0 (Log Viral Load = 1) faster. In average, it took about 14 days of treatment for genotype 1 patients to be free of HCV. For genotype 3 patients, the zero viral load was achieved in about 30 days.
In both cases, the sofosbuvir+daclatasvir combo was proven to be very effective in curing Hepatitis C.
However, some patients who show zero viral load at the end of treatment (EOT), will in some weeks again suffer from Hepatitis C. Some of HCV viruses were not destroyed and evaded detection; without treatment they will multiply and we will again see their presence via measuring the viral load.
In the graph below, we see the efficacy of treatment at 2 distinctive time points:

At the end of the treatment, the resulting cure rate for the most prominent treatment regimens was 99.6%. Out of 236 patients treated, only 1 still showed a non-zero viral load at the end of the treatment. The viral load of the rest 235 patients was zero.
4 weeks after treatment, however, some patient experienced the Hepatitis C recurrence. From the data for GT1 above, we can see that the treatment combo sofosbuvir+ledipasvir went from being 99.2% successful to being 93.7%. This means that in 5.5% of patients treated in such a way, Hepatitis C came back.
On the other hand, sofosbuvir+daclatasvir combo was more successful - the cure rate after 4 weeks fell to 97.3% from 100%. Essentially, only 1 patient with GT1 treated in such a manner suffered from Hepatitis C recurrence.
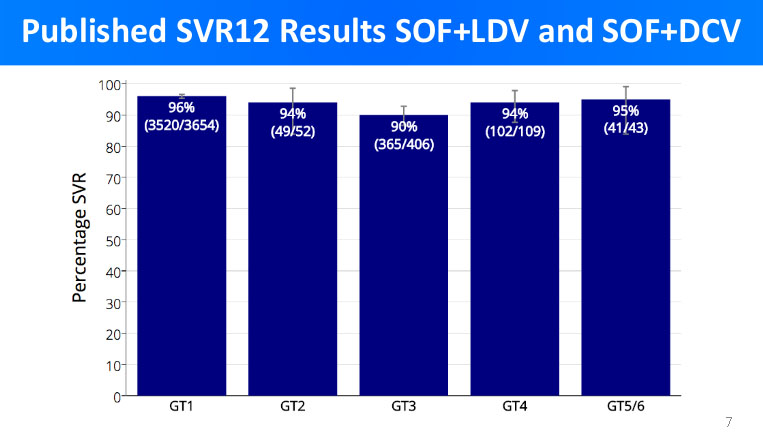
The overall cure rate was observed via parameter SVR12 - sustain virological release measured 12 weeks after the end of treatment. It is a consesus that SVR12 is a true measure of Hepatitis C medication efficacy.
In the graph below are the cure results of branded medications for each genotype.
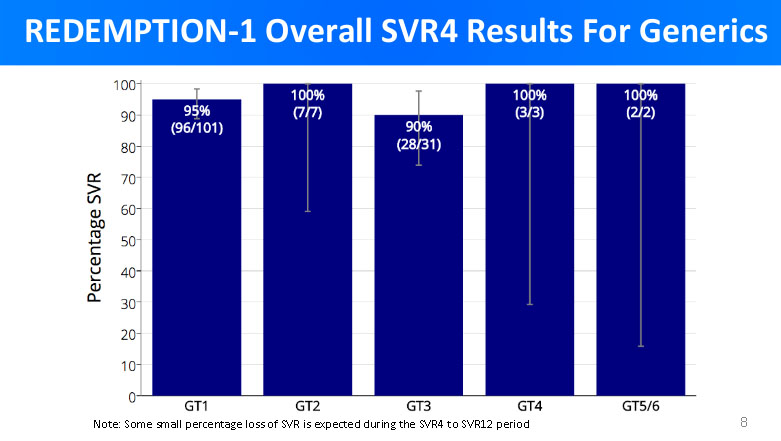
Here are the cure results as obtain with generic DAA treatment in REDEMPTION-1 trials.
By comparing the above graphs, we can see that the cure rates in branded medicines treatment and low-cost generic DAA treatment are very similar.
In order to summarize the results of the trials, Dr. Freeman pointed out 3 very important things to remember:
The successful trial is being praised all over the news. Here are some stories about how the REDEMPTION-1 trials might influence Hepatitis C treatment in the future.
Here below is Dr. Freeman's presentation with added explanations. You can download full version of REDEMPTION-1 clinical trials below (see attachment).

The True Scope of Hepatitis C
As Dr. Freeman neatly put it, the discovery of the modern Hepatitis C drugs that consist of DAA rivals the invention of penicillin by Alexander Fleming in 1928. What is interesting in this analogy, is that penicillin became widely used only in 1942 during the WWII. The DAA treatment is currently available to anyone - with deep enough pocket. Out of estimated 150 million Hepatitis C patients, only 500,000 are treated with the DAA annually. That is less than 1%.Of the Top 5 major causes for infectious disease, Hepatitis C just might be the one that is known the least. However, with the revolutionary DAA treatment making headlines, Hepatitis C awareness is slowly building momentum and the true scope of problems connected with Hepatitis C are becoming more obvious every day; as is the problem of the high prices of branded Hepatitis C treatments.
Top 5 Major Causes of Infectious Diseases:
- HIV/AIDS
- Hepatitis B Virus (HBV)
- Tuberculosis
- Malaria
- Hepatitis C Virus (HCV)
We have had effective treatments for all of these diseases except Hepatitis C for more than a decade. It was in late 2013 that first DAA molecule called sofosbuvir was launched which should in effect greatly improve the world's Hepatitis C statistics by curing Hepatitis C patients. After all, the new Hepatitis C treatments have a cure rate of about 95% with minimal side effects.
Here are some up-to-date statistics on Hepatitis C as presented by Dr. Freeman:
- We have 150 million patients infected with Hepatitis C virus
- We have about 500,000 death connected with Hepatitis C and Hepatitis C related disease
- Even with the effective DAA treatment discovered, we treat only 500,000 patients per year
"If we have 150 million patients, why do we treat only 0.5 million of them? Especially with another 0.5 million patients dying every year."
The answer is pretty obvious as well.
How Big Pharma prices Hepatitis C medicines
The problem is the high cost of treatment.Big Pharma is apt to increase prices of innovative drugs; nonetheless, the Hepatitis C treatment area is another problem altogether because of the very extremes Big Pharma went to price Hepatitis C treatments that are of questionable innovation and are not based on production costs of manufacturing DAA-based pills.
Here is an illustration by Dr. Freeman about how the new standard sofosbuvir-based Hepatitis C treatment costs, and what are the actual production costs for making it.

From the graph above, it is clear that Hepatitis C treatment pricing has nothing to do with the cost of ingredients. Pharmaceutical companies spend $100 to produce Hepatitis C drugs, and sell it for outstanding $84,000 (US price). This incredible margin indirectly translates to 500,000 death every year.
Paradoxically, the discovery of an effective Hepatitis C cure brought more problems than it solved due to how Big Pharma operates.
There is a way around Big Pharma. One of the main objectives of REDEMPTION-1 clinical trials is to provide a scientific basis for treatment with the generic DAAs. It was designed to prove a patient can be cured using the low-cost generic DAA as effectively as with $84,000 priced branded medicines.
Generic DAA and the legality of obtaining it
Generic DAAs are basically the main therapeutic ingredients that branded Hepatitis C medicines are made of. It is important to understand that DAAs hold all of the therapeutic effect; pharmaceutical companies usually add sugars and other non-therapeutical ingredients to transform DAAs in powder form into a pill; a product most of us are used to.According to the study, generic DAAs such as sofosbuvir, ledipasvir and daclatasvir are being mass produced for 1% of the current US price of final products. By using these DAAs for treatment, we can forgo 99% of costs connected with Hepatitis C treatment. However, in many cases legality of using the low-cost generic DAA is put under question. These issues are very important when it comes to obtaining low-cost treatment and were addressed at the International Liver Congress 2016 in Barcelona.
Dr. Freeman pointed out that according to the governing laws of Australia, the UK and other countries, an individual patient has the right to import the maximum of 3-months worth of supply of medicines intended exclusively for personal use.
In the pharmaceutical industry, usually the Big Pharma has patents on therapeutic molecules lawfully giving them monopoly power and privileges. The Hepatitis C treatment are no exception; DAAs such as sofosbuvir, ledipasvir and daclatasvir are all protected by patents.
Nonetheless, World Trade Organization's Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) provides a basis why importing Hepatitis C medicines such as generic DAAs is legal.
According to Article 60 of the said agreement known as De Minimis Imports, the following is stated:
"Members may exclude from the application of the above provisions small quantities of goods of a non-commercial nature contained in travellers' personal luggage or sent in small consignments."
The quotes article states that the TRIPS laws exclude the import of small quantities of non-commercial goods. A Hepatitis C patient travelling out of China with generic DAA pills in his backpack for personal use is an example of practical application of De Minimis Imports article. This also provides a legal basis for fixhepc.com to provide Hepatitis C patients with access to medications and helps them by discussing the treatment on-line.
REDEMPTION-1 Clinical Trials
In order to start the REDEMPTION-1 clinical trials, two key elements were needed:- Credible generic DAAs as treatment.
- Hepatitis C patients as subjects.
Generic DAAs
Generic DAAs were obtained and their quality was evaluated with advanced pharmaceutical techniques such as HPLC, NMR and Mass Spectrometry. To a trained eye, the results obtained (included below) prove that the obtained sofosbuvir DAA has a molecule structure expected from sofosbuvir molecule. This confirms the validity of the generic DAAs used in the REDEMPTION-1 trials.
The peaks in the NMR spector above correspond to specific hydrogen (H) atoms within the molecule of sofosbuvir and confirm the structure of the whole molecule. NMR is a standard technique for obtaining the molecule structure of small therapeutic molecules such as DAAs.
Hepatitis C Patients
In order to determine the effectiveness of the obtained generic DAAs, Hepatitis C patients were recruited via http://fixhepc.com/ website. With the help of Dr. Freeman, more than 400 patients were assisted in making a personal importation of the affordable Hepatitis C medications.
As one can clearly see, the patients from all over the world were included in the trials. Each of them were given an assistance in legally importing the verified generic DAA treatment for an affordable price.
Using the gp2u.com.au Telemedicine Platform each patient was assessed in three stages:
- Before treatment
- During treatment
- After treatment (determining the cure rate via sustain virological release (SVR))
It is well known that there are 6 genotypes of Hepatitis C virus and each of them is treated in a respective manner with a selection of generic DAA. From the poll of over 400 Hepatitis C patients, Dr. Freeman presented the statistical data about the patients involved in the trials.

The statistical data above reveals that the average age of Hepatitis C patient in the trials was 54.4 years. About half of the patients were naive - meaning they received no previous Hepatitis C treatment, and about 30% of them have already suffered liver cirrhosis.
The most predominant genotypes in the trials were genotype 1 (63.9%) and genotype 3 (27.5%), and the most predominant manner of treatment was sofosbuvir+ledipasvir (45.8%) and sofosbuvir+daclatasvir (42.6%).
REDEMPTION-1 Trials Results
The objective of the trials was to evaluate the safety and efficacy of generic DAA treatment and compare them with published data from Phase III clinical trials of existing branded Hepatitis C treatments.The main parameter for measuring the efficacy of Hepatitis C treatment is the decrease in the viral load over time.
In the graph below, we can see the comparison of how quickly does the HCV viral load decrease in patients treated with the generic DAA. Added to the graph are the results of Phase III clinical studies of how quickly did the HCV viral load decreased in patients using branded medicines.

Reading the graph, it is important to see that when Log Viral Load reaches 1, there is no more HCV virus present (since log(1)=0). The results of REDEMPTION-1 trials clearly indicate that the decrease in viral load in patients treated with generic DAA is as quick, or in some cases even quicker, than in patients treated with branded medications. This confirms the premises that the efficacy of generic DAA treatment is comparable to that of the treatment with branded medications.
Here is a more detailed look of how generic DAA treatment combination sofosbuvir+daclatasvir is fighting off HCV in patients with genotype 1 and genotype 3.

As we can see, the viral load of genotype 1 patients has decreased to 0 (Log Viral Load = 1) faster. In average, it took about 14 days of treatment for genotype 1 patients to be free of HCV. For genotype 3 patients, the zero viral load was achieved in about 30 days.
In both cases, the sofosbuvir+daclatasvir combo was proven to be very effective in curing Hepatitis C.
What was the cure rate in REDEMPTION-1 Trials?
A parameter that is observed when we want to determine if a patient is cured of Hepatitis C, is the viral load (HCV RNA). If we cannot detect the genetic material RNA of HCV in the performed test (this means that the content of HCV RNA is below the lower limit of quantification (LLOQ)), we count such a patient as cured.However, some patients who show zero viral load at the end of treatment (EOT), will in some weeks again suffer from Hepatitis C. Some of HCV viruses were not destroyed and evaded detection; without treatment they will multiply and we will again see their presence via measuring the viral load.
In the graph below, we see the efficacy of treatment at 2 distinctive time points:
- At the end of treatment (left columns)
- 4 weeks after the end of treatment (right columns)

At the end of the treatment, the resulting cure rate for the most prominent treatment regimens was 99.6%. Out of 236 patients treated, only 1 still showed a non-zero viral load at the end of the treatment. The viral load of the rest 235 patients was zero.
4 weeks after treatment, however, some patient experienced the Hepatitis C recurrence. From the data for GT1 above, we can see that the treatment combo sofosbuvir+ledipasvir went from being 99.2% successful to being 93.7%. This means that in 5.5% of patients treated in such a way, Hepatitis C came back.
On the other hand, sofosbuvir+daclatasvir combo was more successful - the cure rate after 4 weeks fell to 97.3% from 100%. Essentially, only 1 patient with GT1 treated in such a manner suffered from Hepatitis C recurrence.
The main result of REDEMPTION-1 Trials - EFFICACY
The REDEMPTION-1 trials main objective is to scientifically prove that the generic DAA medicines patients can purchase for about $1,000 are equally successful in curing Hepatitis C as branded medications for which the US patients have to pay $84,000 or more.The overall cure rate was observed via parameter SVR12 - sustain virological release measured 12 weeks after the end of treatment. It is a consesus that SVR12 is a true measure of Hepatitis C medication efficacy.
In the graph below are the cure results of branded medications for each genotype.

Here are the cure results as obtain with generic DAA treatment in REDEMPTION-1 trials.

By comparing the above graphs, we can see that the cure rates in branded medicines treatment and low-cost generic DAA treatment are very similar.
This is the confirmation of REDEMPTION-1 trials objective.
Accordingly, we see less than 6% difference in cure rates between branded medicines and low-cost generic DAA; what is more, in more cases the treatment with generic DAA has proven to be even more successful than with the branded medications.
The main result of REDEMPTION-1 Trials - SAFETY
Dr. Freeman also addressed safety evaluation of the generic DAA treatment. As you can see from the presentation slide below, the safety profile of patients included in the study was comparable to the findings of Phase III clinical studies for respective branded medications.
Conclusions of REDEMPTION-1 Trials
The successful REDEMPTION-1 trials have proven that the Hepatitis C treatment with the generic DAA is as effective and safe as with branded medications.In order to summarize the results of the trials, Dr. Freeman pointed out 3 very important things to remember:
- Generic cure for Hepatitis C is as effective as branded medications, and it only costs $1,000.
- Given $200 costs of production of sofosbuvir+daclatasvir API, the possibility of $200 Hepatitis C cure is now (not in the future)
- There are 150 million Hepatitis C patients in the world. Without low-cost affordable treatment the future of majority of Hepatitis C patients looks like this (check the photo below)

The successful trial is being praised all over the news. Here are some stories about how the REDEMPTION-1 trials might influence Hepatitis C treatment in the future.
- http://www.eurekalert.org/pub_releases/2016-04/eaft-lgd040816.php
- http://ilc-congress.eu/news/
- http://www.aidsmap.com/page/3050874/
- http://hepatitiscnewdrugs.blogspot.si/2016/04/generic-direct-acting-antivirals-for.html
- http://www.medscape.com/viewarticle/862085
Published in
Blogs
Tuesday, 19 April 2016 18:26
Dr. Freeman's Presentation at 2016 International Liver Congress - DAA treatment works like a charm!
The International Liver Congress is a 5-day event where scientists and medical experts from all over the globe come up to speed with what is new in the latest liver research. Arguably, it is the biggest event connected with liver diseases in general, and has the biggest impact factor. This year, more than 10,000 scientific and medical experts met at The International Liver Congress 2016 in Barcelona, Spain. The spotlight of the congress was on what new research says about the possibility of treating liver diseases, and the center was taken by Hepatitis C - the ongoing high prices of Hepatitis C treatments were challenged and alternatives presented.
Dr. Freeman of FixhepC Buyers Club gave one of the most convincing presentations by tackling the most important issue in Hepatitis C treatment today - the high prices of branded drugs coming from Gilead Sciences, Abbvie, BMS and MSD. From the data gathered during large-scale clinical trials with DAA treatments, it is evident, as Dr. Freeman himself pointed out, that patients who cannot afford more than $80,000 for branded Hepatitis C drugs can be treated with DAA with equal success rate. The upside of DAA treatment is the low cost - Hepatitis C patients from all over the world can be cured for as little as $1000.
By proving the generic DAA treatments to be as successful as branded treatment, Dr. James Freeman defined a clear role of generic medicines in Hepatitis C treatment area.
One look at the table of the most expensive prescription drugs in the US will tell you why Hepatitis C treatment sell, and they sell for ridiculously high prices.
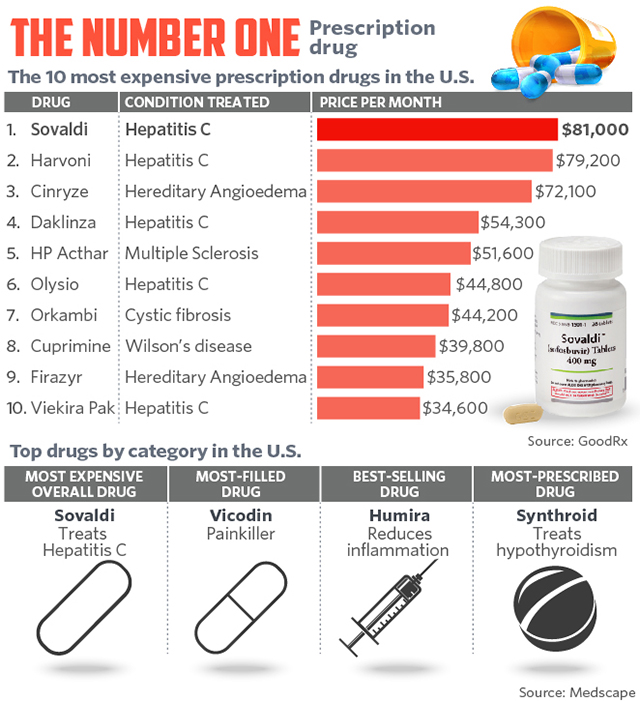
However, for everybody who can afford Hepatitis C treatments like Sovaldi and Harvoni, there are at least 10 people in the US and around the world who cannot even phantom how to raise $80,000 for the treatment without which they will in all probability die.
Dr. Freeman's idea was simple but has a profound effect: Why not use these low-cost DAA, from which branded Hepatitis C treatments are made off, to treat Hepatitis C?
With the redemption trials, Dr. Freeman went out to establish how well do the DAA cure Hepatitis C in comparison with branded drugs such as Sovaldi and Harvoni. The upside, of course, is a huge reduction in Hepatitis C treatment cost. If the DAA work, a patient with Hepatitis C would no longer be asked to pay more than $80,000 for the treatment; he or she will have an alternative option to be treated with DAA for about $1,000. In such a way, millions of Hepatitis C patients all over the world can get an access to the treatment because of it's financial availability.
For the purposes of the trials, the low-cost DAA such as sofosbuvir, ledipasvir, daclatasvir and ribavirin were legally imported and tested with modern analytical techniques such as HPLC, NMR and mass spectrometry. Patients involved in redemption trials came from all over the world - US, UK, Australia, Canada, Europe, SE Asia and Africa.
“Our interim data suggests a potential solution for Hepatitis C patients in areas where treatment access has been restricted as a result of the high prices demanded for branded treatment,” said Dr. James Freeman, the lead author of the study. “At the price level of generic direct-acting antivirals, treating the entire global Hepatitis C epidemic could be financially feasible. Furthermore, if a patient is cured of Hepatitis C, there is evidence for improved survival, and lower risks of liver cancer and liver cirrhosis and cured patients could return to work, delivering further economic benefits to society.”
Trial's results clearly indicate that generic DAA treatment is successful in overall 95% cases in patients with the most common genotype 1. More importantly, this is in line with how well branded medications performed in their respective Phase III clinical trials. This serves as a proof that being treated with banded medication will yield the same results as with generic DAA treatment - however, by choosing the later, you will save $80,000. Not only that, all the patients who cannot afford to fetch up $80,000 will now have an excellent fighting chance by choosing the $1,000 DAA treatment.
Results:
The cure rate for sofosbuvir + ledipasvir (branded medicine: Harvoni) in genotype 1 Hepatitis C patients is 93%.
The cure rate for sofosbuvir + daclatasvir (branded medicine: Sovaldi + Daklinza) in genotype 1 Hepatitis C patients is 97%.
“Across all genotypes, the SVR rate was 94% after treatment with generic DAAs. This indicates that generic DAAs can deliver the same success rates as branded equivalents, but at a price which is 1/100th of the current cost,” explained Dr. James Freeman.
Today everybody infected with HIV is treated with very effective and affordable treatment.
If there was one defining moment in history that changed our view of HIV from a deadly disease to an easily-treatable one, it was a 2004 Lancet published study that proved the same for HIV as Dr. Freeman demonstrated for HCV - Generic medications work as effectively as the originator's medication for a fraction of the cost.
Because of the impact factor the International Liver Congress has, we don't need only to hope for Hepatitis C to be as easily treatable as HIV in 15 years - we can count on it. From an average patient to the US Senate, everybody is pushing for lower cost of Hepatitis C drugs; but as of yet the Big Pharma could not be reasoned with.
Thankfully to Dr. Freeman we don't need Big Pharma to lower the costs any more; now we have a definitive proof that the low-cost generic DAA treatment is equally successful as the drugs Big Pharma wants to sell for above $80,000. We can do it for $1,000 and in such a way we can help everybody - not only 6-figure income people in the US, but a farmer in Australia or a sheep herder in Egypt as well.
“There is a clear role for generic treatments such as these for people with Hepatitis C across the world. The implications of increased availability of these drugs could be enormous, presenting more people with the possibility of a ‘cure’ for what is often a debilitating condition,” said Professor Laurent Castera, EASL Secretary General.
We will give you an inside scoop on the details of the study from Dr. Freeman's presentation in our next blog post. If there is one thing to remember from all of this, it is that when Dr. Freeman was done with the presentation a few light bulbs went off above scientific and medical experts heads - the idea that we can cure the whole planet of Hepatitis C, and we can actually afford to pay for it is a reasonable one.
In the video below you can see the highlights from the International Liver Congress 2015. The video for 2016 highlights is currently in production.
https://www.youtube.com/watch?v=btubKE6OZYg
Dr. Freeman of FixhepC Buyers Club gave one of the most convincing presentations by tackling the most important issue in Hepatitis C treatment today - the high prices of branded drugs coming from Gilead Sciences, Abbvie, BMS and MSD. From the data gathered during large-scale clinical trials with DAA treatments, it is evident, as Dr. Freeman himself pointed out, that patients who cannot afford more than $80,000 for branded Hepatitis C drugs can be treated with DAA with equal success rate. The upside of DAA treatment is the low cost - Hepatitis C patients from all over the world can be cured for as little as $1000.
By proving the generic DAA treatments to be as successful as branded treatment, Dr. James Freeman defined a clear role of generic medicines in Hepatitis C treatment area.
Dr. Freeman's Idea
The International Liver Congress was always a very notable event; however, in recent years the interest and people coming to the event started picking up tremendously. Obtaining a new Hepatitis C cure is a part of the reason why; the other part is that by inventing a very successful Hepatitis C cure, this treatment area became very profitable for everybody who is selling branded Hepatitis C treatments.One look at the table of the most expensive prescription drugs in the US will tell you why Hepatitis C treatment sell, and they sell for ridiculously high prices.

However, for everybody who can afford Hepatitis C treatments like Sovaldi and Harvoni, there are at least 10 people in the US and around the world who cannot even phantom how to raise $80,000 for the treatment without which they will in all probability die.
Generic Hepatitis C market
The secrets of the success of modern Hepatitis C treatments are the newly found active molecules in branded medicines. These active molecules, which carry all the therapeutic effect, are known as direct-acting antivirals or DAA for short. Because how the Big Pharma works, the DAA such as sofosbuvir, ledipasvir and daclatasvir are readily available in pharmaceutically-based manufacturing companies in India and China.Dr. Freeman's idea was simple but has a profound effect: Why not use these low-cost DAA, from which branded Hepatitis C treatments are made off, to treat Hepatitis C?
With the redemption trials, Dr. Freeman went out to establish how well do the DAA cure Hepatitis C in comparison with branded drugs such as Sovaldi and Harvoni. The upside, of course, is a huge reduction in Hepatitis C treatment cost. If the DAA work, a patient with Hepatitis C would no longer be asked to pay more than $80,000 for the treatment; he or she will have an alternative option to be treated with DAA for about $1,000. In such a way, millions of Hepatitis C patients all over the world can get an access to the treatment because of it's financial availability.
DAA Redemption Trials
The purpose of DAA redemption trials was to establish how well do the DAA treat Hepatitis C. We know the cure rate of the majority of branded Hepatitis C treatments is above 90% according to respective Phase III clinical trials done by Gilead Sciences, Abbvie, MSD and BMS.For the purposes of the trials, the low-cost DAA such as sofosbuvir, ledipasvir, daclatasvir and ribavirin were legally imported and tested with modern analytical techniques such as HPLC, NMR and mass spectrometry. Patients involved in redemption trials came from all over the world - US, UK, Australia, Canada, Europe, SE Asia and Africa.
“Our interim data suggests a potential solution for Hepatitis C patients in areas where treatment access has been restricted as a result of the high prices demanded for branded treatment,” said Dr. James Freeman, the lead author of the study. “At the price level of generic direct-acting antivirals, treating the entire global Hepatitis C epidemic could be financially feasible. Furthermore, if a patient is cured of Hepatitis C, there is evidence for improved survival, and lower risks of liver cancer and liver cirrhosis and cured patients could return to work, delivering further economic benefits to society.”
Trial's results clearly indicate that generic DAA treatment is successful in overall 95% cases in patients with the most common genotype 1. More importantly, this is in line with how well branded medications performed in their respective Phase III clinical trials. This serves as a proof that being treated with banded medication will yield the same results as with generic DAA treatment - however, by choosing the later, you will save $80,000. Not only that, all the patients who cannot afford to fetch up $80,000 will now have an excellent fighting chance by choosing the $1,000 DAA treatment.
Results:
The cure rate for sofosbuvir + ledipasvir (branded medicine: Harvoni) in genotype 1 Hepatitis C patients is 93%.
The cure rate for sofosbuvir + daclatasvir (branded medicine: Sovaldi + Daklinza) in genotype 1 Hepatitis C patients is 97%.
“Across all genotypes, the SVR rate was 94% after treatment with generic DAAs. This indicates that generic DAAs can deliver the same success rates as branded equivalents, but at a price which is 1/100th of the current cost,” explained Dr. James Freeman.
The Implications of Successful DAA Redemption Trials
About 15 years ago, instead of Hepatitis C, we were dealing with high prices and resulting lack of availability for HIV drugs. People were dying not because of the lack of treatment but because the treatment was too expensive for most of them to afford.Today everybody infected with HIV is treated with very effective and affordable treatment.
If there was one defining moment in history that changed our view of HIV from a deadly disease to an easily-treatable one, it was a 2004 Lancet published study that proved the same for HIV as Dr. Freeman demonstrated for HCV - Generic medications work as effectively as the originator's medication for a fraction of the cost.
Because of the impact factor the International Liver Congress has, we don't need only to hope for Hepatitis C to be as easily treatable as HIV in 15 years - we can count on it. From an average patient to the US Senate, everybody is pushing for lower cost of Hepatitis C drugs; but as of yet the Big Pharma could not be reasoned with.
Thankfully to Dr. Freeman we don't need Big Pharma to lower the costs any more; now we have a definitive proof that the low-cost generic DAA treatment is equally successful as the drugs Big Pharma wants to sell for above $80,000. We can do it for $1,000 and in such a way we can help everybody - not only 6-figure income people in the US, but a farmer in Australia or a sheep herder in Egypt as well.
“There is a clear role for generic treatments such as these for people with Hepatitis C across the world. The implications of increased availability of these drugs could be enormous, presenting more people with the possibility of a ‘cure’ for what is often a debilitating condition,” said Professor Laurent Castera, EASL Secretary General.
We will give you an inside scoop on the details of the study from Dr. Freeman's presentation in our next blog post. If there is one thing to remember from all of this, it is that when Dr. Freeman was done with the presentation a few light bulbs went off above scientific and medical experts heads - the idea that we can cure the whole planet of Hepatitis C, and we can actually afford to pay for it is a reasonable one.
In the video below you can see the highlights from the International Liver Congress 2015. The video for 2016 highlights is currently in production.
https://www.youtube.com/watch?v=btubKE6OZYg
Published in
Blogs
Tagged under
Thursday, 14 April 2016 19:12
$300 Hepatitis C Cure Could be Available Within 2 Years
14th April 2016 - The world renown International Liver Congress 2016 started yesterday. One of the current highlights is a study by DNDi (Drugs for Neglected Diseases initiative) which introduced a new ravidasvir and sofosbuvir combo for treating Hepatitis C.
According to clinical studies done on 300 patients in Egypt, the new Hepatitis C combo is 100% successful in curing Hepatitis C. What is more, DNDi predicts this medicine will be available in the US, Europe and Japan for - brace yourself - less than $300.
Sovaldi was a stellar achievement and raised the cure rate in Hepatitis C patients from 50% to over 90%. But what made the headlines was it's price point - $80,000 for a single treatment. This transformed Hepatitis C treatment area to one of the most lucrative and profitable areas Big Pharma can find themselves in.
The extreme pricing of Sovaldi led to the extreme pricing of other Hepatitis C medicines which were launched after Sovaldi. Viekira Pak, for example, was launched by AbbVie with a price $83,320 per treatment. In fact, if you check the most expensive drug on the planet list, you're bound to find all Hepatitis C drugs on it and not one of them has a price tag less than $50,000 per treatment.
This is why DNDi's study is so important. It seems that the drug combo they develop is working even better than what Big Pharma came up with, and they are willing to give it to patients for mere $300. This is more than 99% price reduction in Hepatitis C treatment.
DNDi is a non-profit organisation and factors only the manufacturing costs and the costs of running the DNDi into the final cost of medicines. This is how the $300 price tag can be achieved. One has to ask if Gilead has the same costs, why does it charge $94,500 for Harvoni in the US? That means that $300 goes to the manufacturing, and $94,200 is counted as net profit from each and every patient.

By now pretty much everybody knows the story about how Martin Shkreli lifted the prices of HIV medications from $13.50 to $750 per pill. DNDi is doing just the opposite; they will try to reduce $1,000 per pill standard to $3.50 per pill.
DNDi is of an opinion that the new Hepatitis C drugs for $300 will be available in Egypt within 12 months. On the other hand, they are starting to conduct clinical trials in Malaysia and Thailand where patients have different underlying genetic characteristics. Upon successful trials, we are be expected to see Hepatitis C cure for $300 within 18 to 24 months. The drugs will be available in developing countries as well as developed countries. However, bringing such low-priced medicines to markets dominated by pharmaceutical giants such as Gilead Sciences, Abbvie, BMS and MDS might be challenging.
Patients are currently paying more than $1,000 per pill of Sovaldi or Harvoni, and they usually need 84 of them. Pharco Pharmaceutical is expected to produce and sell the drugs that have the same or even better effect on Hepatitis C for $3.50 per pill.
The answer what to do: it depends from patient to patient. The most basic idea is to consult with your doctor about waiting for the new medication. It might suit your wallet, but if your liver deteriorate too much during this period, the size of the wallet won't matter any more.
According to clinical studies done on 300 patients in Egypt, the new Hepatitis C combo is 100% successful in curing Hepatitis C. What is more, DNDi predicts this medicine will be available in the US, Europe and Japan for - brace yourself - less than $300.
$300 instead of $80,000 for curing Hepatitis C
Hepatitis C was not a big market for pharmaceutical industry prior to 2013. Gilead Sciences, a pharmaceutical giant, launched Sovaldi (400mg sofosbuvir) in December 2013; this is when everything changed. Hepatitis C became one of the most important therapeutic areas for Big Pharma.Sovaldi was a stellar achievement and raised the cure rate in Hepatitis C patients from 50% to over 90%. But what made the headlines was it's price point - $80,000 for a single treatment. This transformed Hepatitis C treatment area to one of the most lucrative and profitable areas Big Pharma can find themselves in.
The extreme pricing of Sovaldi led to the extreme pricing of other Hepatitis C medicines which were launched after Sovaldi. Viekira Pak, for example, was launched by AbbVie with a price $83,320 per treatment. In fact, if you check the most expensive drug on the planet list, you're bound to find all Hepatitis C drugs on it and not one of them has a price tag less than $50,000 per treatment.
This is why DNDi's study is so important. It seems that the drug combo they develop is working even better than what Big Pharma came up with, and they are willing to give it to patients for mere $300. This is more than 99% price reduction in Hepatitis C treatment.
DNDi is a non-profit organisation and factors only the manufacturing costs and the costs of running the DNDi into the final cost of medicines. This is how the $300 price tag can be achieved. One has to ask if Gilead has the same costs, why does it charge $94,500 for Harvoni in the US? That means that $300 goes to the manufacturing, and $94,200 is counted as net profit from each and every patient.

By now pretty much everybody knows the story about how Martin Shkreli lifted the prices of HIV medications from $13.50 to $750 per pill. DNDi is doing just the opposite; they will try to reduce $1,000 per pill standard to $3.50 per pill.
Ravidasvir and Sofosobuvir combo clinical study in Egypt
DNDi teamed up with Egyptian pharmaceutical company Pharco Pharmaceuticals to conduct clinical studies in Egypt. 300 genotype 4 Hepatitis C patients were included in the study, and the cure rate is between 98%-100%. This showed principle investigators that one does not need to use ribavirin in order to successfully cure genotype 4 Hepatitis C.DNDi is of an opinion that the new Hepatitis C drugs for $300 will be available in Egypt within 12 months. On the other hand, they are starting to conduct clinical trials in Malaysia and Thailand where patients have different underlying genetic characteristics. Upon successful trials, we are be expected to see Hepatitis C cure for $300 within 18 to 24 months. The drugs will be available in developing countries as well as developed countries. However, bringing such low-priced medicines to markets dominated by pharmaceutical giants such as Gilead Sciences, Abbvie, BMS and MDS might be challenging.
What are Hepatitis C patients to do?
An average Hepatitis C patient should right now be asking the following question 'Do I buy Harvoni for $94,500 or wait two years and buy this new Hepatitis C combo for $300?'. The difference is ground-breaking.Patients are currently paying more than $1,000 per pill of Sovaldi or Harvoni, and they usually need 84 of them. Pharco Pharmaceutical is expected to produce and sell the drugs that have the same or even better effect on Hepatitis C for $3.50 per pill.
The answer what to do: it depends from patient to patient. The most basic idea is to consult with your doctor about waiting for the new medication. It might suit your wallet, but if your liver deteriorate too much during this period, the size of the wallet won't matter any more.
Published in
Blogs
Tagged under
Monday, 11 April 2016 18:49
Sovaldi was originally priced at $36,000 - Why do Hepatitis C patient have to pay more than $80,000 for it?
By now pretty much everybody knows which common disease will your wallet hate the most: Hepatitis C.
Let us look at a list of the most expensive drugs in the US. There are top 10 on the list; can you guess how many of them are Hepatitis C treatments?
We will explain an extraordinary story why all of these Hepatitis C drugs found their way on the list. In 2011, the highest expected price point for Hepatitis C cure was $36,000. Now patients are paying more than $80,000 for the privilege. What happened?
The real prices of the most expensive drugs in the US.

As you can see in this summary by Medscape, 5 out of 10 most expensive drugs in the US are Hepatitis C treatments. Gilead Sciences takes the 1st and the 2nd price with their extremely highly priced sofosbuvir-based treatments Sovaldi and Harvoni.
Pharmaceutical companies often try to justify high costs of drugs because of all the research and development that went into finding the innovative new drugs. Admittedly, R&D costs are high and rising, but Gilead Sciences was a bit shy to use R&D costs and innovation as an excuse for the extreme pricing of Sovaldi. Why?
If there was a single identifiable moment when Hepatitis C patients should scream at the tops of their lungs, it was the 2011 purchase of Pharmasset by one Gilead Science Inc.. Gilead paid $11.2 billion for a company with the ground-breaking Hepatitis C molecule. All they had to do, is just bring the research to conclusion, launch it and Hepatitis C patients would be well off, knowing they have an affordable medicine.
Instead, Gilead Sciences made a giant correction in the price of new Hepatitis C treatment. They raised what was supposed to be $36,000 cure into $80,000 cure. Given that patients don't really have a choice, Gilead put their bets on a sole premise that patients would rather pay $80,000 than die. And it paid off in a big way.
Gilead Sciences stock after Pharmasset purchase
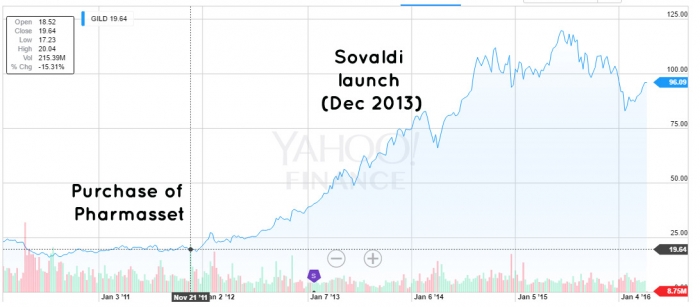
When Gilead bought Pharmasset, its shares were trading below $20 and the company's market capitalisation was less than $30 billion. Gilead was a small-to-middle-player in the biotech industry.
In two years it took for Gilead to turn sofosbuvir molecule into Sovaldi medication, its shares were trading 300% higher at $80, and had a market capitalisation near $100 billion. Sovaldi has single-handedly made Gilead Sciences into a biotech giant it is today.
What has Gilead actually done?
Bought another company and harvested the fruits of their labor. It's just like a normal person going to the store, buying a dishwasher and selling the washed dishes for $1,000 a plate. Gilead just brought it home in a big way.
Sofosbuvir enables Hepatitis C treatments to be even more than 95% successful in curing Hepatitis C. However, despite the miracle effect, the molecule is not something all that new. If a medical chemist would compare sofosbuvir with acyclovir, standard antiviral, he would see some difference but practically the same bone structure of the molecule. Sofosbuvir is just a bit tweaked, that's all.
Acyclovir (left) and Sofosbuvir (right) - Molecules are different but the principle of how molecular structure affects Hepatitis C is the same
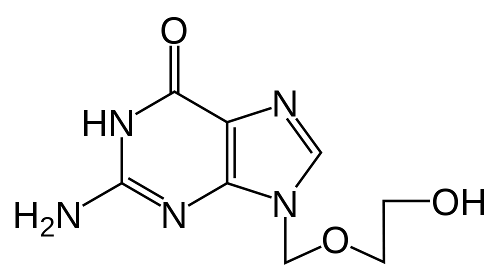
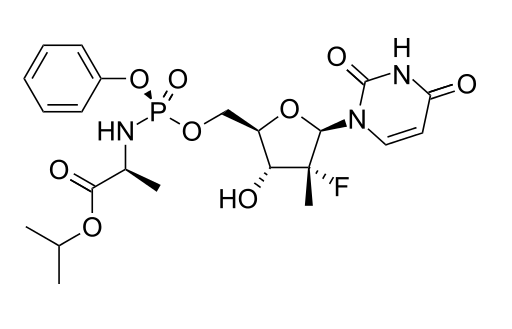
In the developed world, this tweaking means innovation. However, India did not find the Gilead's new sofosbuvir molecule all that different from other similar antiviral active ingredients. They stated that Gilead failed to showcase a significant innovation and this is why India did not grant patent rights to Gilead for their Hepatitis C drugs. Gilead had to struck a deal with Indian pharmaceutical companies to generate its Hepatitis C drugs and sell it for about $1,000. One has to ask - if they can sell Sovaldi for $1,000 and still turn a sizable profit, why are they selling Sovaldi for more than $80,000 in the US? How much profit are they making by selling drugs to people who would die without them?
Let us look at a list of the most expensive drugs in the US. There are top 10 on the list; can you guess how many of them are Hepatitis C treatments?
We will explain an extraordinary story why all of these Hepatitis C drugs found their way on the list. In 2011, the highest expected price point for Hepatitis C cure was $36,000. Now patients are paying more than $80,000 for the privilege. What happened?
The real prices of the most expensive drugs in the US.

As you can see in this summary by Medscape, 5 out of 10 most expensive drugs in the US are Hepatitis C treatments. Gilead Sciences takes the 1st and the 2nd price with their extremely highly priced sofosbuvir-based treatments Sovaldi and Harvoni.
How Sovaldi Came to the US Market
Sovaldi and Harvoni are marketed by a pharmaceutical giant Gilead Sciences. They were the ones who got the market authorisation approval by the FDA in December, 2013, and launched Sovaldi - the first all-oral Hepatitis C cure. Without a doubt, the first effective Hepatitis C cure was a game changer. It didn't only change how we perceived Hepatitis C but how we see pharmaceutical industry as a whole. The reason? Just look at the current Sovaldi price point - above $80,000 per a single treatment.Pharmaceutical companies often try to justify high costs of drugs because of all the research and development that went into finding the innovative new drugs. Admittedly, R&D costs are high and rising, but Gilead Sciences was a bit shy to use R&D costs and innovation as an excuse for the extreme pricing of Sovaldi. Why?
R&D Costs for Sovaldi were very low
The reason is because Gilead Scienced didn't do all the R&D job themselves; they didn't even do the majority of it. The necessary R&D was done by Pharmasset, a pharmaceutical company that was bought by Gilead because of its wonderful sofosbuvir molecule (Sovaldi consists of 400 mg sofosbuvir pills). Pharmasset has done the majority of work, and in their SEC filing in 2011 they projected that when the sofosbuvir-based drug will be finished and launched, 'a U.S. base rate of $36,000 per course of treatment' will be applicable. So how did we come to $80,000 price tag for Sovaldi?If there was a single identifiable moment when Hepatitis C patients should scream at the tops of their lungs, it was the 2011 purchase of Pharmasset by one Gilead Science Inc.. Gilead paid $11.2 billion for a company with the ground-breaking Hepatitis C molecule. All they had to do, is just bring the research to conclusion, launch it and Hepatitis C patients would be well off, knowing they have an affordable medicine.
Instead, Gilead Sciences made a giant correction in the price of new Hepatitis C treatment. They raised what was supposed to be $36,000 cure into $80,000 cure. Given that patients don't really have a choice, Gilead put their bets on a sole premise that patients would rather pay $80,000 than die. And it paid off in a big way.
Gilead Sciences stock after Pharmasset purchase

When Gilead bought Pharmasset, its shares were trading below $20 and the company's market capitalisation was less than $30 billion. Gilead was a small-to-middle-player in the biotech industry.
In two years it took for Gilead to turn sofosbuvir molecule into Sovaldi medication, its shares were trading 300% higher at $80, and had a market capitalisation near $100 billion. Sovaldi has single-handedly made Gilead Sciences into a biotech giant it is today.
What has Gilead actually done?
Bought another company and harvested the fruits of their labor. It's just like a normal person going to the store, buying a dishwasher and selling the washed dishes for $1,000 a plate. Gilead just brought it home in a big way.
Lack of Innovation
The key active ingredient that goes into Sovaldi and Harvoni is sofosbuvir; this is why Pharmasset was bought for $11.2 billion. In hindsight, combined Sovaldi and Harvoni sales will probably peak over $20 billion in sales - if Gilead has done something right, it was its purchase of Pharmasset.Sofosbuvir enables Hepatitis C treatments to be even more than 95% successful in curing Hepatitis C. However, despite the miracle effect, the molecule is not something all that new. If a medical chemist would compare sofosbuvir with acyclovir, standard antiviral, he would see some difference but practically the same bone structure of the molecule. Sofosbuvir is just a bit tweaked, that's all.
Acyclovir (left) and Sofosbuvir (right) - Molecules are different but the principle of how molecular structure affects Hepatitis C is the same


In the developed world, this tweaking means innovation. However, India did not find the Gilead's new sofosbuvir molecule all that different from other similar antiviral active ingredients. They stated that Gilead failed to showcase a significant innovation and this is why India did not grant patent rights to Gilead for their Hepatitis C drugs. Gilead had to struck a deal with Indian pharmaceutical companies to generate its Hepatitis C drugs and sell it for about $1,000. One has to ask - if they can sell Sovaldi for $1,000 and still turn a sizable profit, why are they selling Sovaldi for more than $80,000 in the US? How much profit are they making by selling drugs to people who would die without them?
Published in
Blogs
Tagged under
Wednesday, 06 April 2016 20:04
18 Ways To Cope With Hepatitis C
Hepatitis C is a disease that many people have to live with for years. Even with the newly discovered treatment, some people still choose to forgo therapy due to high prices of medications. We have prepared a list of things every Hepatitis C patient can do to help himself or herself.
The best way to get rid of Hepatitis C is of course to get the treatment with DAAs such as sofosbuvir, ledipasvir, daclatasvir and ribavirin. FixHep C Buyers Club offers these treatments for less than $2,000, with a kind help of Dr. Freeman; the original price of these medications can cost up to $100,000.
Here are some ideas how to help yourself if you are suffering from Hepatitis C.
Picking some things from this list might help you to keep a positive outlook as well as keeping your body healthy.
The best way to get rid of Hepatitis C is of course to get the treatment with DAAs such as sofosbuvir, ledipasvir, daclatasvir and ribavirin. FixHep C Buyers Club offers these treatments for less than $2,000, with a kind help of Dr. Freeman; the original price of these medications can cost up to $100,000.
Here are some ideas how to help yourself if you are suffering from Hepatitis C.
18 Ways To Cope With Hepatitis C
- Join a support group. Talking to other people about the same problems is a very effective method to cope with any disease. Many HIV, addicts, alcoholics and so on are finding it as a means of acceptance, and Hepatitis C patients are recommended to find local Hepatitis C meetings and attend them.
- Stay away from alcohol, tobacco, excess caffeine and illicit drugs. If you can’t quit, cut back or ask for help.
- Keep your vaccinations current. Be immunized against hepatitis A and B.
- Aim for 7 to 9 hours of sleep every night.
- Strive to be as physically active as you can be on a regular basis. There are many choices, such as walking, bicycling, gardening, dancing, swimming, stretching, Yoga, Tai Chi or strength training. Just keep moving.
- Maintain a healthy weight.
- Eat a low fat, high fiber diet. Include fruit, vegetables, and whole grains. Avoid trans-fatty acids and saturated fats.
- Balance rest and activity.
- Cultivate a positive attitude.
- Avoid or reduce stress.
- Engage in activities that give you pleasure and make you laugh.
- Choose activities that stimulate your brain.
- Engage your spirit in meaningful ways, such as meditation, a walk in the woods, prayer.
- Learn to laugh at yourself.
- Drink at least 8 glasses of water every day.
- Maintain friendships and social contacts.
- Help others. Volunteer your time.
- Remind yourself of the things in your life to be grateful for or that you appreciate.
Picking some things from this list might help you to keep a positive outlook as well as keeping your body healthy.
Published in
Blogs
Tagged under
