Marko
Monday, 04 April 2016 19:46
Is The Availability of Hepatitis C Drugs from India threatened?
Where does India come in in the pharmaceutical industry?
This is how drug industry works.New drugs are being developed by big pharmaceutical corporations in the developed world (mostly the US and Europe). When such originator drugs are launched, they are usually very expensive and patients and healthcare insurances have to pay even 100s of dollars per dollar of costs of actually making the drug.
Hepatitis C treatment area is a great example of that: Gilead Sciences developed an active molecule sofosbuvir (in hindsight, they bought a company that developed sofosbuvir, and invested in clinical studies to bring it to the market). They registered a drug called Sovaldi that consists of 400mg sofosbuvir pills, and they are selling a single such pill for about $1000 in the US. The manufacturing costs to create such pill are a lot less than $10. But, after all, this is how pharmaceutical industry works.
India - 'A pharmacy for the developing world'
India is special because they enable access to expensive drugs by creating generic versions of such drugs. This is why India is dubbed 'A pharmacy for the developing world'. They are known to produce low-cost medicines that are also being sold for low prices. Gilead may produce low-cost medicines (they will pay pennies on the dollar for production costs) but you'll never see them selling it for low prices.
Patents are how big pharmaceutical corporations secure monopoly in the drug business and is the reason why they can sell drugs at such high prices. What makes India so special is not only that they are capable of producing drugs; it is that they have a special patent law that is not so much in favour of big corporations than in the rest of the world - and this is exactly the law that Europe and the US want them to change.
Indian patent law is designed specifically to prevent ever greening. Ever greening is the practice of pharma business big players to extend or get new patents on old drugs - in many times by making small changes in the active molecule or package it is some new way. In such a way, the drug effectiveness is not significantly increased - but the company sales of the drugs are huge because they can get a patent for it as a novel drug and sell it at high prices. Well, that can't be done in India.
Here is an example of ever greening. AstraZeneca, the second biggest pharmaceutical company in the UK, has been selling a drug containing omeprazole for some years (omeprazole is a treatment for acid reflux in the stomach). When a patent on omeprazole was about to expire and the drugs they were selling for lets say $50 would be reduced to $10 due to generic competition, they 'invented' a new active molecule called esomeprazole. This is how they could not only maintain $50 drug prices, but even increase the price to lets say $70.
The problem? Look at the difference between the drugs.
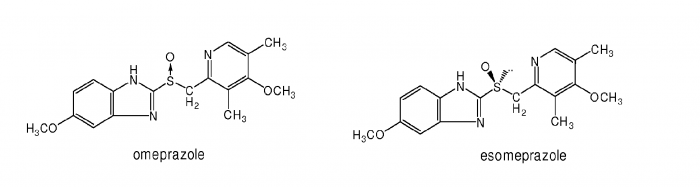
One does not need to be an expert in drug design to see that the molecules are practically identical; there is just a bit of a difference in the middle of the molecule (S-O bond). The treatment effects of both molecules are comparable; albeit esomeprazole was proven to be slightly better. AstraZeneca sold esomeprazole up till 2015 - at that point they were selling about $5 billion of esomeprazole per year. Without it, they would lose tens of billions of dollars that would remain in the pockets of their patients.
India's free trading agreement with Europe
In Hepatitis C world, the story of Australian Greg Jefferys is well known. He was a Hepatitis C patient who couldn't afford $100,000 for the medicine Gilead Sciences was selling. He is one of the first who flew to India, bought generic Sovaldi for $1,200 and got cured. This is an example how every Hepatitis C patient can benefit from India's drug industry, and of course how the big corporations in the developed world can fail in extorting huge sums of money from people who know will die without the drugs the big pharma is selling.With the recent renewal of India's free trade agreement with Europe, the practices of India's patent law offices are coming into question. If these laws would be changed to the laws in developed countries, there would be no more low-cost medicines for patients. There would be no more cheap Sovaldi and Harvoni for Hepatitis C patients.
The good news is that India is now likely to bow down to Europe.
'People will die' if India bows to Europe
The statement was made by Gregg Jefferys who experiences the first hand the harshness that big pharma possess and the solution that India's generic pharmaceutical industry presents.Dr. Prabhash Ranjan, Prof. of Trade Law at South Asia University, is of an option that the patent law is not likely to be changed in India. India simply has too much to lose. Because of all the benefits of low-cost medicines, India is reaping a lot of success with the so-called 'Hepatitis C tourism' - welcoming people from all around the world who can't pay $100,000 for Hepatitis C drugs. They come to India for a week or two, get the drugs for $1000 or so, and are cured within 3 months.
We will let you know how everything turns out. In the meantime, be sure to contact us and get the Hepatitis C drugs from India while the loophole is still open.
Published in
Blogs
Monday, 28 March 2016 21:07
A New Wave of Hepatitis C Patients Under the Age of 30 in the US
Hepatitis C is a disease that mostly affects people after 40 years of age. Being an asymptomatic disease, Hepatitis C virus (HCV) can be hiding in the liver for more than a decade without apparent effects. This gives us an indication about when Hepatitis C patients were infected - not in older age, but in many cases before the age of 30 via the use of illicit injectable drugs. According to this next graph, we shall see why we have to be concerned about raising Hepatitis C awareness and informing our youth about the danger they may face later on in life.
Rates of newly confirmed Hepatitis C patients in two age groups: The young (15-24 years) and everybody else, in Massachusetts (2002-2009)
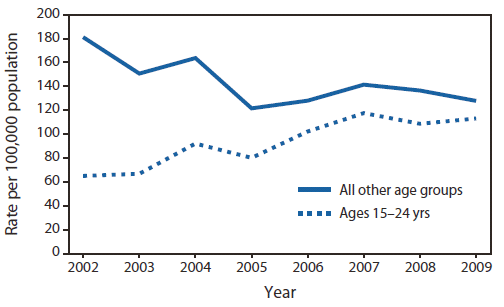
If we look at the 'All other age groups' curve, we see that the rate of people newly infected with Hepatitis C is steadily dropping from 0.18% of the population to 0.12%.
The disconcerting curve is the second curve. We see that the incidence of Hepatitis C patients in the US state of Massachusetts rose for almost 100% in 7 years. This indicated that there is a new wave of Hepatitis C patients on the horizon; the upside here is that by properly informing our youth about the dangers of injecting illegal drugs via needles - this is the most common way of Hepatitis C transmission - we can drastically decrease the incidence of new Hepatitis C patients.
The survey data was gathered by Centers for Disease Control and Prevention for 34 states, and 634 people were interviewed about drug use.
The most common transmission of Hepatitis from person to person is via sharing an injectable needle. With more than 50% of Hepatitis C patients being infected in such a way, it is a major source of recovery if we tap into it in the right way. Namely, if we remove the infected needles out of equation, Hepatitis C spread can be stopped very effectively.
However, the problem is that more and more young people are using injectable drugs. This issue is being addressed for many decades now but the results, as we clearly see from the numbers of people still injecting themselves with opioids, are disconcerning . While stopping drug use is a very important cause, it is not necessarily needed to stop the spread of Hepatitis C.
One way to break the chain reaction that is currently happening in the US is to provide clean needles. Many do see it as a kind of an incentive that even promotes drug use, but with regard to Hepatitis C, the solution, if implemented correctly, was of much benefit. There are no chances people will get infected if they use clean needles.
Another solution is to 'tag' Hepatitis C patients, similarly to HIV positive patients. However, this approach is met with ethical dilemmas and even if it were implemented thoroughly would not yield as effective results as complete stop of drug use.
If you do the maths, you see that there are more than 20.000 additional cases of future chronic Hepatitis C patients, just due of the new increase.
Considering the high costs of treatment (for example Harvoni, most prominent Hepatitis C medication, costs $94,500 for standard treatment in the US), these additional cases alone will cost more $1 billion to treat. Now this is a subsancial figure that can make you think twice before injecting yourself with a non-sterilized needle.
Rates of newly confirmed Hepatitis C patients in two age groups: The young (15-24 years) and everybody else, in Massachusetts (2002-2009)

If we look at the 'All other age groups' curve, we see that the rate of people newly infected with Hepatitis C is steadily dropping from 0.18% of the population to 0.12%.
The disconcerting curve is the second curve. We see that the incidence of Hepatitis C patients in the US state of Massachusetts rose for almost 100% in 7 years. This indicated that there is a new wave of Hepatitis C patients on the horizon; the upside here is that by properly informing our youth about the dangers of injecting illegal drugs via needles - this is the most common way of Hepatitis C transmission - we can drastically decrease the incidence of new Hepatitis C patients.
Hepatitis C infection is not slowing down - it is speeding up
According to the article published in Oxford Journals, the rate of young people below the age of 30 contracting Hepatitis C was steadily increasing during 2006 to 2012. Here are some interesting results based on national surveillance in the US:- 13% annual increase in reported Hepatitis C cases for young people in non urban areas
- 5% annual increase in reported Hepatitis C cases for young people in urban areas
- In 75% of cases, patients admit they used illicit injectable drugs via needle (opioids)

The survey data was gathered by Centers for Disease Control and Prevention for 34 states, and 634 people were interviewed about drug use.
Drug use is propelling the rise of Hepatitis C
Looking at the CDCP data, one will find out that it's not Hepatitis C that is the issue - it is improper drug use.The most common transmission of Hepatitis from person to person is via sharing an injectable needle. With more than 50% of Hepatitis C patients being infected in such a way, it is a major source of recovery if we tap into it in the right way. Namely, if we remove the infected needles out of equation, Hepatitis C spread can be stopped very effectively.
However, the problem is that more and more young people are using injectable drugs. This issue is being addressed for many decades now but the results, as we clearly see from the numbers of people still injecting themselves with opioids, are disconcerning . While stopping drug use is a very important cause, it is not necessarily needed to stop the spread of Hepatitis C.
Clean needles may be enough
Hepatitis C is something drug users may not be concerned about at first because it's effects will show only after 10 years or more. Nonetheless, the core of the problem with Hepatitis C are 'infected needles'. These are needles that were used by a Hepatitis C positive person at first but were later used by a Hepatitis C negative person without prior sterilization - in such a way, we get two patients from one patient, and the chain reaction continues.One way to break the chain reaction that is currently happening in the US is to provide clean needles. Many do see it as a kind of an incentive that even promotes drug use, but with regard to Hepatitis C, the solution, if implemented correctly, was of much benefit. There are no chances people will get infected if they use clean needles.
Another solution is to 'tag' Hepatitis C patients, similarly to HIV positive patients. However, this approach is met with ethical dilemmas and even if it were implemented thoroughly would not yield as effective results as complete stop of drug use.
Toll paid by the society for illegal drug use by 15-24 year olds
According to demography of the US, there are more than 40 million American aged between 15 and 24 years old. As we have seen from graph above, the incidence between 2002 and 2009 rose from about 0.06% to 0.012%. How many more young Americans are suffering from Hepatitis C?If you do the maths, you see that there are more than 20.000 additional cases of future chronic Hepatitis C patients, just due of the new increase.
Considering the high costs of treatment (for example Harvoni, most prominent Hepatitis C medication, costs $94,500 for standard treatment in the US), these additional cases alone will cost more $1 billion to treat. Now this is a subsancial figure that can make you think twice before injecting yourself with a non-sterilized needle.
Published in
Blogs
Tagged under
Friday, 25 March 2016 00:09
Example of a country where Hepatitis C spread is at an alarming rate - Pakistan
We have secured a viable cure for Hepatitis C with a sofosbuvir-based regimen. However, healing the existing patients is not enough. We also have to approach systematic treatment from another angle - reduce Hepatitis C spread.
In the developed world, the newly discovered treatment is already taking effect and the number of Hepatitis C patients finally started to decrease. However, the most problematic countries that produce more and more Hepatitis C patients without a systematic healthcare system eliminating the HCV, are third world countries. Today we will look at the example of just such a country - Pakistan.
Pakistan is the country where the number of Hepatitis C patients is increasing at an alarming rate. These five practices speak all about it.
In injection practice, alarming thing is not the injection itself but unhygienic reuse of syringes. There are cases where doctors have had limited supply of syringes for a whole week. This unhygienic practice let the infected person transfer the hepatitis virus silently to the other person.
Doctor-to-patient transmission is something the developed world is not immune to as well. A few weeks ago we wrote a story about a UK based NHS doctor who potentially infected more than 8,000 patients with HCV.
Spread of Hepatitis C all over the world
They administer infected syringes, use non-scientific methods and prescribe sub-standard medicines. In many ways, quakes create an environment where infected person transfer the virus to other healthy person. These self-styled doctors do not recommend their patients basic tests and administer injection at their own whims. Many patients with chronic hepatitis C have had a history of quake treatment at an early stage.
Central blood bank is not effective enough to cater the demands of the whole country. When patients is a critical situation, the relatives have to procure blood on their own. This critical situation puts the question of hepatitis C free blood aside and results in unchecked transfusion of infected blood to the patient- leading to more cases of hepatitis C.
First, it is a complicated and prolonged.
Second, it is costly and patients have to go for many costly tests during the treatment. Patient prefers to go for herbal medicines, as herbal treatment is less costly and patient-friendly. This method of treatment brings temporary relief in some cases but destroys the liver completely leading to complete liver failure.
In the developed world, the newly discovered treatment is already taking effect and the number of Hepatitis C patients finally started to decrease. However, the most problematic countries that produce more and more Hepatitis C patients without a systematic healthcare system eliminating the HCV, are third world countries. Today we will look at the example of just such a country - Pakistan.
Pakistan is the country where the number of Hepatitis C patients is increasing at an alarming rate. These five practices speak all about it.
1. Injecting a silent killer
Administering interferon through intra-vascular injection is still a common mode of treatment in Pakistan. It is embedded deep down in medical culture and practices. In rural areas, the predominant symbol of doctor is the person holding a mighty injection. In many ways, this image of a doctor is interesting and opposite to the universal image of doctor wearing a white coat and holding a stethoscope around her neck.In injection practice, alarming thing is not the injection itself but unhygienic reuse of syringes. There are cases where doctors have had limited supply of syringes for a whole week. This unhygienic practice let the infected person transfer the hepatitis virus silently to the other person.
Doctor-to-patient transmission is something the developed world is not immune to as well. A few weeks ago we wrote a story about a UK based NHS doctor who potentially infected more than 8,000 patients with HCV.
Spread of Hepatitis C all over the world

2. Quakes are part of problem not part of solution
Quakes are a sort of physicians in Pakistan. They do not study medicine or surgery but they do practice both. They are mostly working or retired paramedics but they act like real doctors and start their own clinics. Quakes are least concerned about preventive measures and hygienic standards.They administer infected syringes, use non-scientific methods and prescribe sub-standard medicines. In many ways, quakes create an environment where infected person transfer the virus to other healthy person. These self-styled doctors do not recommend their patients basic tests and administer injection at their own whims. Many patients with chronic hepatitis C have had a history of quake treatment at an early stage.
3. Blood transfusion is a serious thing!
In Pakistan, the practice of blood transfusion is not controlled as in the developed world. With the exception of few hospitals in big cities, most of the hospitals in the country lack facilities to storage donor blood.Central blood bank is not effective enough to cater the demands of the whole country. When patients is a critical situation, the relatives have to procure blood on their own. This critical situation puts the question of hepatitis C free blood aside and results in unchecked transfusion of infected blood to the patient- leading to more cases of hepatitis C.
4. Desi Dawai (herbal treatment) does not work for every disease
There is a general misconception in hepatitis C treatment. People take interferon treatment as an unwelcome approach for two reasons.First, it is a complicated and prolonged.
Second, it is costly and patients have to go for many costly tests during the treatment. Patient prefers to go for herbal medicines, as herbal treatment is less costly and patient-friendly. This method of treatment brings temporary relief in some cases but destroys the liver completely leading to complete liver failure.
5. Diagnosis requires proper tests
A huge chunk of the population does not have access to sophisticated virus tests. They even do not know until the last stage that they have been carrying a virus for more than 20 years. The silent killer remains hidden in the body of a patient until it turns into a monster and becomes fatal.
Published in
Blogs
Tuesday, 22 March 2016 20:13
Hepatitis C Burden and Distribution in Africa
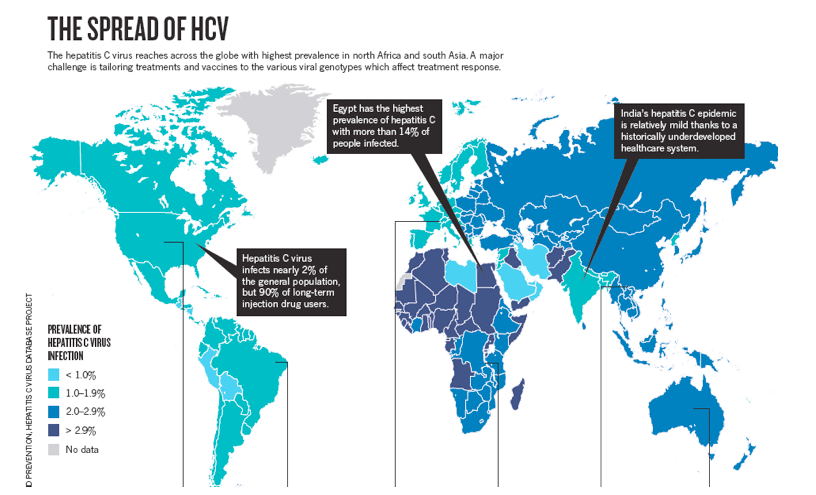
The Hepatitis C virus (HCV) infects people all over the world but Africa is a special case. The virus causes a chronic liver disease and is among the leading factors of liver problems in the world. The statistics from the World Health Organisation (WHO) reveal that an estimated one hundred and seventy million people in the entire world are infected with around four million reported new infections every year.
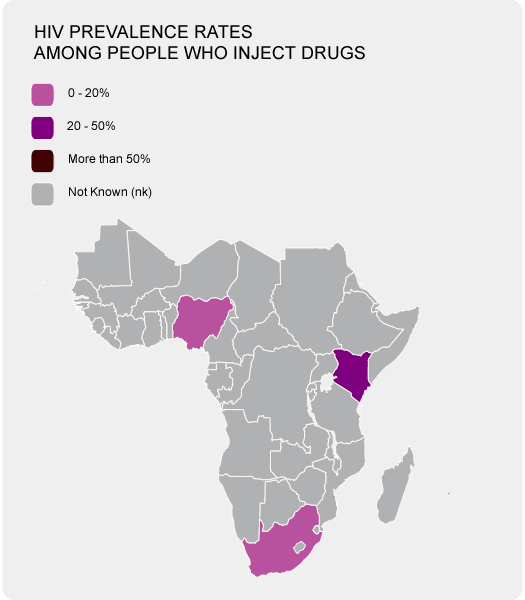
An estimated prevalence in Africa is at 5.3% with Egypt having a prevalence of 17.5%. Egypt has one of the highest reported prevalences. This is a result of Egypt's government not agreeing to use a somewhat expensive diagnostic tests to determine if donor blood is infected with HCV. In the US, for example, these kinds of tests have been around from 1992 and are vital for limiting the spread of the disease.
The prevalence of the HCV appears to increase with age as the highest reported cases are those people who are aged forty years and above.
As you see in the photo on your right, the majority of African countries have troubles coming up with a tangible figure as far as HIV prevalence rates are concerned. Getting these information for HPV are even more elusive.
There are analytical tests that can detect the HPV in donor blood. However, in Africa, only a small percentage (about a fifth of all blood sampled) is tested for the HCV. This is due to the costs involved in laboratory testing.
In Africa, many of way of Hepatitis C transmission are not controlled. Not testing donor blood is just a tip of the iceberg; the unprotected sex due to lack of condoms and so on are only increasing the chances of HPV spreading.
HIV/AIDS has a higher prevalence in Africa as well making a pathway for HPV to spread. On the other hand, the lack of needles and systemic check of drug use is sprouting more and more cases of HPV via needle injections.
HCV awareness campaigns and counselling services that reduce the risks to exposure and infection control practices should also be implemented in Africa. Hopefully with so much funds being generated by the big corporations who sell Hepatitis C medicines, at least some part will go to helping less fortunate.
Gilead Sciences has partly done that by giving licenses to produce generic version of Sovaldi and Harvoni but in Africa stopping the spread is the real issue.
The spread of HPV is a vicious circle. Luckily the spread is not as quick as in other viruses (especially those who spread by inhalation) but the number are steadily rising despite us having the cure for Hepatitis C. Africa should be calling for a systematic solution but as in many other cases, doing something systematic and long-lasting in Africa is very far fetched.
Why is Africa special as far as Hepatitis C is concerned
In Africa alone the figures are at an astonishing thirty-two million infections making it the highest reported data in the world. With these statistics, HCV remains highly prevalent and infectious in nature with the virus infection remaining under-reported and under-diagnosed in Africa apart from a few countries like Egypt who have reliable data.Prevalence of Hepatitis C in Africa

An estimated prevalence in Africa is at 5.3% with Egypt having a prevalence of 17.5%. Egypt has one of the highest reported prevalences. This is a result of Egypt's government not agreeing to use a somewhat expensive diagnostic tests to determine if donor blood is infected with HCV. In the US, for example, these kinds of tests have been around from 1992 and are vital for limiting the spread of the disease.
The prevalence of the HCV appears to increase with age as the highest reported cases are those people who are aged forty years and above.
As you see in the photo on your right, the majority of African countries have troubles coming up with a tangible figure as far as HIV prevalence rates are concerned. Getting these information for HPV are even more elusive.
Problem of not testing donor blood for HPV
HCV is transmitted by various methods such as blood and blood products, organs and tissues, unsafe medical procedures, intravenous drug injection, sexual intercourse, through body piercings and vertical transmission i.e. mother infecting a fetus. A problematic method of transmission is by blood donation.There are analytical tests that can detect the HPV in donor blood. However, in Africa, only a small percentage (about a fifth of all blood sampled) is tested for the HCV. This is due to the costs involved in laboratory testing.

Spread of HPV in Africa
The populations that are at most risk are those who intravenously inject themselves with drugs, HIV/AIDS patients who are undergoing the hemodialysis procedure, organ transplantation and blood transfusion patients and surprisingly even the medical staff themselves can be infected if they acquire injuries caused by used needles. Other groups of people at risk include sexually active adults with several partners and children who are born to infected mothers. Also, the data available on HCV disclose that patients with the chronic liver disease tend to have a high prevalence.In Africa, many of way of Hepatitis C transmission are not controlled. Not testing donor blood is just a tip of the iceberg; the unprotected sex due to lack of condoms and so on are only increasing the chances of HPV spreading.
HIV/AIDS has a higher prevalence in Africa as well making a pathway for HPV to spread. On the other hand, the lack of needles and systemic check of drug use is sprouting more and more cases of HPV via needle injections.
What is being done to prevent HPV spread in Africa?
The primary prevention strategies of the HCV include testing and proper testing of blood, plasma, tissue, semen and organ donors. The virus can also be inactivated by-products derived from blood plasma. However, the strategies that work in the developed world are very rarely used in Africa.HCV awareness campaigns and counselling services that reduce the risks to exposure and infection control practices should also be implemented in Africa. Hopefully with so much funds being generated by the big corporations who sell Hepatitis C medicines, at least some part will go to helping less fortunate.
Gilead Sciences has partly done that by giving licenses to produce generic version of Sovaldi and Harvoni but in Africa stopping the spread is the real issue.
The spread of HPV is a vicious circle. Luckily the spread is not as quick as in other viruses (especially those who spread by inhalation) but the number are steadily rising despite us having the cure for Hepatitis C. Africa should be calling for a systematic solution but as in many other cases, doing something systematic and long-lasting in Africa is very far fetched.
Published in
Blogs
Tagged under
Tuesday, 15 March 2016 19:13
Hepatitis C History and Why Baby Boomers have the highest incidence of Hepatitis C infection
I just opened a textbook for medical students from 1980s, doing a research on Hepatitis C. Here's the interesting thing: When looking for liver diseases, in particular Hepatitis, the table of content only lists Hepatitis A and Hepatitis B. Where is Hepatitis C?
"There are some evidence that another virus (Hepatitis C?) is present in the liver."
This is the quote about Hepatitis C from 1980s. About 30 years later, we found a very successful cure consisting of sofosbuvir, ledipasvir, daclatasvir and ribavirin treatment options, for a lethal disease. Let us have a look at a brief history of Hepatitis C.
Some scientist predict that the subforms of Hepatitis C (genotypes) originated about 500 years ago in West Africa.
Nonetheless, the spread of disease hundreds of years ago was much slower than today. Hepatitis C is a transmitted via blood-to-blood contact, and 500 years ago there were no blood transfusions or needle injections which are the leading cause of Hepatitis C transmission in today's world.
Evidence suggest that more than 90% of patients diagnosed with NANB were actually Hepatitis C patients.
A year later, the screening of blood banks began but the method of Hepatitis C virus detection in blood used for transfusion was perfected in 1992. From that point on, only 1 blood bag out of 2 million is statistically infected with Hepatitis C.
However, prior to 1992, there was no way of detecting Hepatitis C in donated blood and it is speculated that about 1 blood bag out of 200 was infected by Hepatitis C. Due to these blood transfusion prior to 1992, an approximate 300,000 Americans were infected by Hepatitis C via transfusion. These include many war veterans.
Particularly the generation of Baby Boomers were infected by receiving the Hepatitis C infected blood.
The first Hepatitis C treatment was actually discovered earlier than 1989. In 1957, scientist discovered a treatment that was used more than 50 years for Hepatitis treatment - interferon. Primarily developed against Hepatitis A and B, it was also used to treat Hepatitis C patients.
The general treatment protocol was to inject 3 million units of interferon, three times a week for 48 weeks. Sustained virological response rates (negative viral load 6 months post-treatment) were approximately 9% for genotype 1 and 30% for genotypes 2 and 3.
Interferon name actually comes from its ability to 'interfere' with viral replication. The interference is not only useful against HCV but against other viruses as well.
However, the cure rate was only 50%. Compared to sofosbuvir-based regimen of today, interferon is a much inferior treatment and is likely not going to be used for Hepatitis C treatment anymore.
During the following years, there has been some improvement in Hepatitis C treatment such as pegylated interferon and addition of some antiviral molecule to the regimen. However, the big breakthrough came with the DAA - direct acting antivirals such as sofosbuvir, ledipasvir and daclatasvir.
In 2013, the FDA has approved the first modern Hepatitis C treatment and the golden age for Hepatitis C treatment discovery started. Here are all the drugs that were recently approved for treatment of various genotypes of chronic Hepatitis C.
The problem and the reason why so much media is featuring the stories about Hepatitis C, is the price of the treatment. Not a single of drug above cost less than $50,000 in the US. Even US Senate became involved but the prices are still extremely high.
From interferon injections to a pill-a-day regimen
With only the cost being the major problem, we do welcome you to contact us at FixHepC Buyers Club and we will help you get Hepatitis C medications for a very affordable price.
"There are some evidence that another virus (Hepatitis C?) is present in the liver."
This is the quote about Hepatitis C from 1980s. About 30 years later, we found a very successful cure consisting of sofosbuvir, ledipasvir, daclatasvir and ribavirin treatment options, for a lethal disease. Let us have a look at a brief history of Hepatitis C.
History of Hepatitis C Treatment
It is almost impossible to know how long has Hepatitis C virus been around - we don't even have blood samples from lets say 50 years ago which could confirm the existence of HCV in 1960s.Ancient History of Hepatitis C
The nature of many viruses inclines us to a conclusion that Hepatitis C virus has been around for a very long time. In fact, a close relative of HCV called HGV/GBV-C originated in primates about 35 million years ago, according to some theories.Some scientist predict that the subforms of Hepatitis C (genotypes) originated about 500 years ago in West Africa.
Nonetheless, the spread of disease hundreds of years ago was much slower than today. Hepatitis C is a transmitted via blood-to-blood contact, and 500 years ago there were no blood transfusions or needle injections which are the leading cause of Hepatitis C transmission in today's world.
Hepatitis A and B - The first suggestion there might be Hepatitis C
In 1960s and 1970s, scientists developed blood tests to identify hepatitis B (1963) and hepatitis A (1973), but many of the blood samples taken for post-transfusion illness tested negative for hepatitis A and hepatitis B. However, in addition to A and B, a virus in the liver was discovered. Because it was neither Hepatitis A or B virus, the newly discovered virus was named NANB Hepatitis. This stands for non-A non-B Hepatitis.Evidence suggest that more than 90% of patients diagnosed with NANB were actually Hepatitis C patients.
Discovery of Hepatitis C virus and Blood Testing
The virus itself was identified in 1989. The discovery is credited to the investigators from the Centers for Disease Control.A year later, the screening of blood banks began but the method of Hepatitis C virus detection in blood used for transfusion was perfected in 1992. From that point on, only 1 blood bag out of 2 million is statistically infected with Hepatitis C.
However, prior to 1992, there was no way of detecting Hepatitis C in donated blood and it is speculated that about 1 blood bag out of 200 was infected by Hepatitis C. Due to these blood transfusion prior to 1992, an approximate 300,000 Americans were infected by Hepatitis C via transfusion. These include many war veterans.
Particularly the generation of Baby Boomers were infected by receiving the Hepatitis C infected blood.
Discovery of first Hepatitis C treatment - Interferon

The first Hepatitis C treatment was actually discovered earlier than 1989. In 1957, scientist discovered a treatment that was used more than 50 years for Hepatitis treatment - interferon. Primarily developed against Hepatitis A and B, it was also used to treat Hepatitis C patients.
The general treatment protocol was to inject 3 million units of interferon, three times a week for 48 weeks. Sustained virological response rates (negative viral load 6 months post-treatment) were approximately 9% for genotype 1 and 30% for genotypes 2 and 3.
Interferon name actually comes from its ability to 'interfere' with viral replication. The interference is not only useful against HCV but against other viruses as well.
Inclusion of Ribavirin to Hepatitis C treatment
In 1998, FDA approved Rebetron - a treatment for Hepatitis C that included not only interferon but ribavirin antiviral drug molecule as well. This drastically increased the cure rate to about 30% for genotype 1 and 60% for genotype genotypes 2 and 3.However, the cure rate was only 50%. Compared to sofosbuvir-based regimen of today, interferon is a much inferior treatment and is likely not going to be used for Hepatitis C treatment anymore.
During the following years, there has been some improvement in Hepatitis C treatment such as pegylated interferon and addition of some antiviral molecule to the regimen. However, the big breakthrough came with the DAA - direct acting antivirals such as sofosbuvir, ledipasvir and daclatasvir.
Hepatitis C Treatment Breakthrough
2012 was a year for DAAs. Several new Hepatitis C treatment candidates were very promising in Phase III clinical trials, yielding an outstanding 90-100% cure rates. Several pharmaceutical companies were involved in drugs development, each with its own DAA and regimen.In 2013, the FDA has approved the first modern Hepatitis C treatment and the golden age for Hepatitis C treatment discovery started. Here are all the drugs that were recently approved for treatment of various genotypes of chronic Hepatitis C.
- Sovaldi (sofosbuvir) by Gilead Sciences
- Harvoni (sofosbuvir and ledipasvir) by Gilead Sciences
- Olysio (simeprevir) by Janssen
- Daklinza (daclatasvir) by Bristol-Myers Squibb
- Viekira Pak (ombitasvir, paritaprevir, ritonavir and dasabuvir) by AbbVie
- Zepatier (elbasvir and grazoprevir) by Merck Co.
The problem and the reason why so much media is featuring the stories about Hepatitis C, is the price of the treatment. Not a single of drug above cost less than $50,000 in the US. Even US Senate became involved but the prices are still extremely high.
From interferon injections to a pill-a-day regimen

From Hepatitis C discovery to a cure in less than 25 years
Hepatitis C is a serious disease and HCV is a very resilient type of virus. However, it took our combined science less than 25 years to discover and develop the cure - with Sovaldi being approved by FDA in 2013, we finally got a formidable medication against HCV.With only the cost being the major problem, we do welcome you to contact us at FixHepC Buyers Club and we will help you get Hepatitis C medications for a very affordable price.
Published in
Blogs
Tagged under
Thursday, 10 March 2016 20:13
Is Zepatier cost low enough to drive down the Hepatitis C treatment prices?
Hepatitis C treatment prices are one of the most discussed issues in the medical world today. The patients want lower prices. The doctors have spoken against the high prices, and even the US Senate had a hearing about it. But the price of Hepatitis C treatment remained the same even almost 3 years after the first modern treatment - Sovaldi by Gilead Sciences - was introduced.
In January 2016, Merck joined the Hepatitis C treatment market with the launch of Zepatier, a new and effective Hepatitis C treatment. While the Zepatier's cure rates for genotypes 1 and 4 are very comparable with other existing treatments such as Harvoni and Viekira Pak, Zepatier has one thing going for it - a lower price point for treatment. Will the lower Zepatier cost be enough to bring down the cost of other Hepatitis C treatments?
Let us take a look at the Hepatitis C treatment prices.
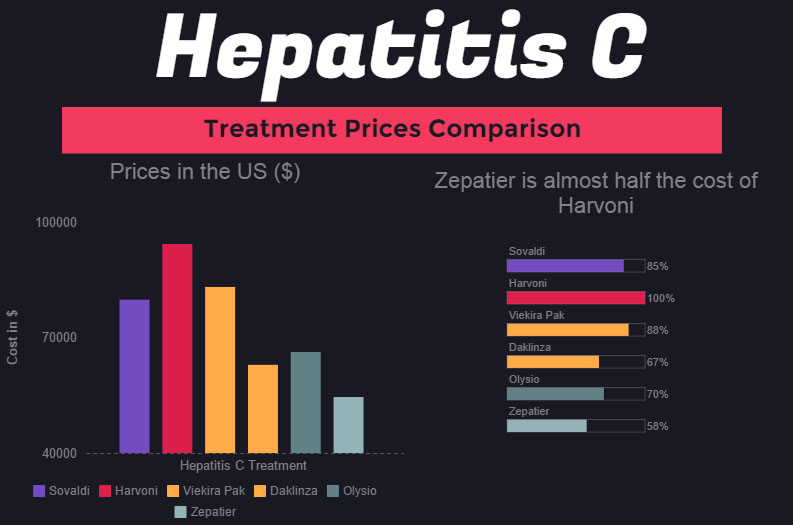
As we see from the graph, the first treatment Sovaldi's price point is $80,000, followed by Harvoni's $94,500 price point - to date, Harvoni cost of standard treatment is the highest. When AbbVie introduced Viekira Pak at $83,319 per treatment, the price of Harvoni didn't decrease - primarily because Harvoni has an advantage over Viekira Pak due to simpler regimen.
Daklinza, introduced by BMS, also didn't manage to decrease the prices of other Hepatitis C treatments. However, the introduction of Zepatier with the lowest price point for modern Hepatitis C treatment at $54,600 might at least do something to decrease the costs. As we see, Zepatier cost is more than 40% lower than that of Harvoni, while the medications can arguably considered as very similar in effect.
1. Gilead offers co-pay program for Hepatitis C patients covering 25% of Harvoni cost
2. Gilead gave licences to several Indian companies to produce generic Sovaldi and Harvoni to be sold in the third world countries
A good news is that majority of Hepatitis C patients can qualify for the Harvoni co-pay program. The price is reduced but it is still more than $70,000 per treatment. Generic Sovaldi and Harvoni are sold for less than $2,000 in India but one has to travel there to get them - this is where FixHepC Buyers Club steps in and helps patients to get it in a convienent way without the need to travel to India and bargain with the doctors and sellers there.
Zepatier is the one we have been waiting for that can change the game. Not only does Zepatier offer a high cure rate for naive patients, the clinical research has revealed that in some patients who were already being unsuccessfully treated with interferon, Zepatier might have better clinical results than both Sovaldi and Harvoni - making Zepatier a contender to be reckoned with.
On top of this, Zepatier price of treatment is $54,600 in the US. In a normal economy, when you have two very comparable goods such as Harvoni and Zepatier, with one being much cheaper than the other, people will opt to go for the cheaper version. In this case, the only way for Harvoni to still be competitive, is to reduce it price and make it more comparable with the price of Zepatier.
Nonetheless, this is the pharmaceutical industry where things are not as simple as they might seem. Harvoni, being already launched for about two years, has build up a reputation as a safe and effective Hepatitis C cure. Zepatier is a new-comes - less known but cheaper. Only time will tell how this plays out but it is a hope of most of Hepatitis C community that the lower cost of Zepatier will help decrease the cost of Sovaldi, Harvoni, Viekira Pak and Daklinza due to the increased competition.
In January 2016, Merck joined the Hepatitis C treatment market with the launch of Zepatier, a new and effective Hepatitis C treatment. While the Zepatier's cure rates for genotypes 1 and 4 are very comparable with other existing treatments such as Harvoni and Viekira Pak, Zepatier has one thing going for it - a lower price point for treatment. Will the lower Zepatier cost be enough to bring down the cost of other Hepatitis C treatments?
Zepatier cost vs the cost of other Hepatitis C treatments
If the voices of patients, doctors and even the US Senate have no effect on how big pharma is pricing Hepatitis C treatments - because this is how capitalism works - will the introduction of Zepatier decrease these prices by presenting major competition? According to general capitalistic economics, the added competition will have a net effect on lowering the prices across the board. But this is pharmaceutical industry with its specifics - can we still count on economic laws to work here?Let us take a look at the Hepatitis C treatment prices.

As we see from the graph, the first treatment Sovaldi's price point is $80,000, followed by Harvoni's $94,500 price point - to date, Harvoni cost of standard treatment is the highest. When AbbVie introduced Viekira Pak at $83,319 per treatment, the price of Harvoni didn't decrease - primarily because Harvoni has an advantage over Viekira Pak due to simpler regimen.
Daklinza, introduced by BMS, also didn't manage to decrease the prices of other Hepatitis C treatments. However, the introduction of Zepatier with the lowest price point for modern Hepatitis C treatment at $54,600 might at least do something to decrease the costs. As we see, Zepatier cost is more than 40% lower than that of Harvoni, while the medications can arguably considered as very similar in effect.
What has been done thus far to decrease Hepatitis C treatment costs?
Gilead Sciences, producer of Sovaldi and Harvoni, and the main player in Hepatitis C treatment market has thus far take two measures to react to the overwhelming pressure from all sides as far as the treatment cost reduction is concerned.1. Gilead offers co-pay program for Hepatitis C patients covering 25% of Harvoni cost
2. Gilead gave licences to several Indian companies to produce generic Sovaldi and Harvoni to be sold in the third world countries
A good news is that majority of Hepatitis C patients can qualify for the Harvoni co-pay program. The price is reduced but it is still more than $70,000 per treatment. Generic Sovaldi and Harvoni are sold for less than $2,000 in India but one has to travel there to get them - this is where FixHepC Buyers Club steps in and helps patients to get it in a convienent way without the need to travel to India and bargain with the doctors and sellers there.
How can Zepatier lower cost effect Hepatitis C treatment prices in the near future?
After Harvoni, Viekira Pak and Daklinza, two very successful Hepatitis C treatments were launched with a notably lower price point. However, Gilead remained unyielding and did nothing about the existing price of Sovaldi and Harvoni, despite the introduction of competition. This may be because Sovaldi and Harvoni have a strategical advantage over the new competition - Viekira Pak has a complex regimen (a patient has to take multiple pills per day) and Daklinza usually involves the use of sofosbuvir (an active ingredient in both Harvoni and Sovaldi), making both of them a tad bit inferior competitors.Zepatier is the one we have been waiting for that can change the game. Not only does Zepatier offer a high cure rate for naive patients, the clinical research has revealed that in some patients who were already being unsuccessfully treated with interferon, Zepatier might have better clinical results than both Sovaldi and Harvoni - making Zepatier a contender to be reckoned with.
On top of this, Zepatier price of treatment is $54,600 in the US. In a normal economy, when you have two very comparable goods such as Harvoni and Zepatier, with one being much cheaper than the other, people will opt to go for the cheaper version. In this case, the only way for Harvoni to still be competitive, is to reduce it price and make it more comparable with the price of Zepatier.
Nonetheless, this is the pharmaceutical industry where things are not as simple as they might seem. Harvoni, being already launched for about two years, has build up a reputation as a safe and effective Hepatitis C cure. Zepatier is a new-comes - less known but cheaper. Only time will tell how this plays out but it is a hope of most of Hepatitis C community that the lower cost of Zepatier will help decrease the cost of Sovaldi, Harvoni, Viekira Pak and Daklinza due to the increased competition.
Published in
Blogs
Tagged under
Monday, 07 March 2016 02:46
Hepatitis C Drugs Insurance Coverage Practices investigated by NY attorney general
Insurance companies are a very important part of the lives of many Hepatitis C patients. With the effective new drugs costing up to $100,000, the majority of people are counting on their insurance companies to foot the bill. However, the staggering prices of Hepatitis C drugs present a crippling cost to many health insurance companies who might have a trick or two in the pocket to unlawfully deny Hepatitis C drug coverage to their clients.
The insurance companies are in many cases the ones who pay for the expensive treatments; but Hepatitis C drugs are giving even them a headache. Some cases of consumers lawsuits were drawn together in Massachusetts, where the local attorney general is continuing an inquiry if the state-operated Medicaid programs are in violation of a federal law by denying Hepatitis C patients access to a proven and effective medicine.
Eric Schneiderman, the NY state attorney general, is picking up the trail - initially, he sent out two subpoenas to insurance companies questioning them about the practices of processing their client's request for the coverage of Harvoni; one of the most successful Hepatitis C drugs with a price tag of $94,500 per standard treatment. Up to date, the total number of subpoenas sent climb to 16. A person familiar with the investigation reported that all the major insurance companies such as Aetna, CareConnect, and EmblemHealth are involved and that Schneiderman is interested in processing documentation regarding the authorization of Hepatitis C drugs; in particular the documentation of Hepatitis C patients who were denied treatment coverage was requested.
New York state Attorney General Eric Schneiderman investigates insurance companies on account of Hepatitis C drugs coverage practices

New York state Attorney General Eric Schneiderman’s office has issued subpoenas to 16 health insurers—which includes all major commercial plans in the state—requesting documents on the companies’ processes for authorising drugs used to treat hepatitis C and documentation on patients who have been denied coverage, said a person familiar with the investigation
Schneiderman believes that more than a thousand of patients have been denied coverage unlawfully. If he is right, insurance companies may well have scammed Hepatitis C patients out of more than $100 million in Hepatitis C drug coverage costs.
The NY attorney general is also challenging some heavy-weights. Harvoni, for example, is not some secondary drug - just last year, Gilead Sciences sold $13,86 billion of Harvoni alone. With more than 150 million patients with Hepatitis C, this is one of the most prominent and best-selling medications in the history. Additionally, insurance companies have a lot to lose if they unlawfully denied Hepatitis C drug coverage to patients in need.
NY Attorney General investigates insurance companies that denied Hepatitis C drugs to their clients
From the arrival of the first modern Hepatitis C treatment, patients and governments alike have been questioning the pricing of such treatments by Hepatitis C drug producers such as Gilead and AbbVie. The US Senate even had a series of hearings about the rising costs of drugs in the last years; the debates were largely derived from the abnormal prices of Hepatitis C drugs such as Sovaldi and Harvoni.The insurance companies are in many cases the ones who pay for the expensive treatments; but Hepatitis C drugs are giving even them a headache. Some cases of consumers lawsuits were drawn together in Massachusetts, where the local attorney general is continuing an inquiry if the state-operated Medicaid programs are in violation of a federal law by denying Hepatitis C patients access to a proven and effective medicine.
Eric Schneiderman, the NY state attorney general, is picking up the trail - initially, he sent out two subpoenas to insurance companies questioning them about the practices of processing their client's request for the coverage of Harvoni; one of the most successful Hepatitis C drugs with a price tag of $94,500 per standard treatment. Up to date, the total number of subpoenas sent climb to 16. A person familiar with the investigation reported that all the major insurance companies such as Aetna, CareConnect, and EmblemHealth are involved and that Schneiderman is interested in processing documentation regarding the authorization of Hepatitis C drugs; in particular the documentation of Hepatitis C patients who were denied treatment coverage was requested.
New York state Attorney General Eric Schneiderman investigates insurance companies on account of Hepatitis C drugs coverage practices

New York state Attorney General Eric Schneiderman’s office has issued subpoenas to 16 health insurers—which includes all major commercial plans in the state—requesting documents on the companies’ processes for authorising drugs used to treat hepatitis C and documentation on patients who have been denied coverage, said a person familiar with the investigation
Hepatitis C cases in New York City

Why is being truthful about Hepatitis C drug coverage a big deal?
Many cases in Hepatitis C world are a life or death situation - and this one is not an exception. If you are relying on the insurance company to cover the costs of Hepatitis C treatment, and you get denied, it is as close as most people will come to a death sentence. If everything is going according to the rules, we call it just; but according to the NY Attorney General something smells fishy about how insurance companies determine if a Hepatitis C patient is rightfully justified to have his or her treatment costs covered. In some cases, patients are denied Hepatitis C drug coverage because their liver is 'too healthy'. Having Hepatitis C is not enough to justify the costs of treatment according to some insurance company policies; in such a case, a patient can wait for his or her liver to be ruined just enough to justify the treatment - which is in itself morally and medically questionable.Schneiderman believes that more than a thousand of patients have been denied coverage unlawfully. If he is right, insurance companies may well have scammed Hepatitis C patients out of more than $100 million in Hepatitis C drug coverage costs.
The NY attorney general is also challenging some heavy-weights. Harvoni, for example, is not some secondary drug - just last year, Gilead Sciences sold $13,86 billion of Harvoni alone. With more than 150 million patients with Hepatitis C, this is one of the most prominent and best-selling medications in the history. Additionally, insurance companies have a lot to lose if they unlawfully denied Hepatitis C drug coverage to patients in need.
Notice for all Hepatitis C patients
The insurance company may cover the costs of your treatment, or not. Before you consider paying $94,500 for Harvoni, contact FixHepC Buyers Club and we will try to help you get the medications you need for less than $2000. Why should you be paying almost $100,000 for the medications that costed less than $1000 to produce?
Published in
Blogs
Tagged under
Wednesday, 02 March 2016 18:09
Sunvepra (100mg asunaprevir) - A New Hepatitis C Drug for difficult-to-treat patients
The last two years or so have been very successful for Hepatitis C treatments. The revolutionary new line of drugs that was registered for chronic Hepatitis C since 2013 feature over 90% cure rates and a pill-per-day regimens. Asunaprevir is the latest drug that could provide an edge especially in patients who are difficult to treat. With a completely new mechanism of action, asunaprevir (the drug is already registered in Japan under the trademark name Sunvepra) might be very useful for the following patients:
For example, the most well-known Hepatitis C drug sofosbuvir works by inhibiting NS5B protein in Hepatitis C virus. Sofosbuvir is not used for treatment by its own; modern treatments fight Hepatitis C virus with 2 or more drug molecules. Harvoni, for instance, includes ledipasvir, an NS5A protein inhibitor, to more effectively fight HCV, and even the most basic Sovaldi (400mg sofosbuvir) regimen includes ribavirin or/and interferon.
Safe to say, it is important to have as many as possible drugs working in different ways to most effectively treat Hepatitis C, and asunaprevir is another useful way how to target HCV. What is important to understand is that viruses are a living matter (albeit scientist disagree if viruses are actually alive or not; they do agree on that they are able to change and adapt to the environment). If you have ever wondered why we have 6 genotypes of Hepatitis C virus, here is your answer - it is this adaptational ability that enables HCV to change and fight against the drugs that are used to fight it.
The solution: Hit HCV with everything we got at the same time. Hepatitis C treatment consists of two or more drugs to attack two or more targets in the virus. Asunaprevir offers us a new way to target the virus that proved to be very resilient to existing drugs.
Asunaprevir's molecular structure

Sunvepra (100mg asunaprevir) is often partnered up with Daklinza (daclatasvir) for treatment. In such a way, daclatasvir can target NS5A protein while at the same time asunaprevir targets NS3. The virus can try to fight each of the drugs by changing its morphology, pumping out the drug, and so on, but if for example it can resist the attack of daclatasvir on NS5A protein, asunaprevir can still finish it off by attacking NS3 protein and impede HCV's ability to replicate.
In an official statement by Bristol-Meyers Squibb about asunaprevir, the chief scientist working on the project, Kazuaki Chayama of Hiroshima University in Japan, explain why asunaprevir was specifically registered in Japan.
“Japan has a unique hepatitis C patient population, many of whom are older and have been unable to take, or respond to, traditional therapies, so we have a real sense of urgency to treat these patients now."
Nonetheless, some speculation about BMS's move to limit the countries in which they registered Sunvepra point out that it may have something to do with limited competition in Japan and Russia, and the problem of lower effectiveness of asunaprevir regimen compared to other Hepatitis C treatments.
- Asunaprevir could benefit patients who didn't respond to prior therapy with Sovaldi, Harvoni, Daklinza, Zepatier and so on
- Asunaprevir could also benefit who were medically ineligible or intolerant to previous treatment
How does Asunaprevir treat Hepatitis C?
We already have several drug molecules for the treatment of Hepatitis C that as asunaprevir are very effective at fighting Genotype 1 cases. These include sofosbuvir, ledipasvir, daclatasvir, ribavirin and so on. One important difference between asunaprevir and these drugs is the novel mechanism of action asunaprevir brings to the table.For example, the most well-known Hepatitis C drug sofosbuvir works by inhibiting NS5B protein in Hepatitis C virus. Sofosbuvir is not used for treatment by its own; modern treatments fight Hepatitis C virus with 2 or more drug molecules. Harvoni, for instance, includes ledipasvir, an NS5A protein inhibitor, to more effectively fight HCV, and even the most basic Sovaldi (400mg sofosbuvir) regimen includes ribavirin or/and interferon.
Safe to say, it is important to have as many as possible drugs working in different ways to most effectively treat Hepatitis C, and asunaprevir is another useful way how to target HCV. What is important to understand is that viruses are a living matter (albeit scientist disagree if viruses are actually alive or not; they do agree on that they are able to change and adapt to the environment). If you have ever wondered why we have 6 genotypes of Hepatitis C virus, here is your answer - it is this adaptational ability that enables HCV to change and fight against the drugs that are used to fight it.
The solution: Hit HCV with everything we got at the same time. Hepatitis C treatment consists of two or more drugs to attack two or more targets in the virus. Asunaprevir offers us a new way to target the virus that proved to be very resilient to existing drugs.
Asunaprevir Mechanism of Action
Asunaprevir (formerly known as BMS-650032) works unlike any other Hepatitis C on the market. The mechanism of action of asunaprevir is to target a specific HCV enzyme called serine protease NS3. This is an enzyme that virus needs for normal functionality and replication, and by blocking it, asunaprevir inhibits HCV ability to grow while immune system swoops in with antibodies to fight off the remaining virus.Asunaprevir's molecular structure

Sunvepra (100mg asunaprevir) is often partnered up with Daklinza (daclatasvir) for treatment. In such a way, daclatasvir can target NS5A protein while at the same time asunaprevir targets NS3. The virus can try to fight each of the drugs by changing its morphology, pumping out the drug, and so on, but if for example it can resist the attack of daclatasvir on NS5A protein, asunaprevir can still finish it off by attacking NS3 protein and impede HCV's ability to replicate.
Asunaprevir in Japan
Sunvepra which consists of 100mg of asunaprevir is currently not registered as widely as other Hepatitis C drugs. The treatment is used primarily in Japan and Russia.In an official statement by Bristol-Meyers Squibb about asunaprevir, the chief scientist working on the project, Kazuaki Chayama of Hiroshima University in Japan, explain why asunaprevir was specifically registered in Japan.
“Japan has a unique hepatitis C patient population, many of whom are older and have been unable to take, or respond to, traditional therapies, so we have a real sense of urgency to treat these patients now."
Nonetheless, some speculation about BMS's move to limit the countries in which they registered Sunvepra point out that it may have something to do with limited competition in Japan and Russia, and the problem of lower effectiveness of asunaprevir regimen compared to other Hepatitis C treatments.
When HCV becomes more and more resistant...
In the case of viral infections such as Hepatitis C, an arsenal of many different drugs can prove very useful in the long term. Viruses such as HCV have outstanding survival mechanisms which can even render a drug that was once had 100% cure rate ineffective. By broadening the number of molecules we have to fight HCV, we are reducing the risk of HCV resistance to drugs. For example, if HCV learns how to render sofosbuvir inactive, we have daclatasvir, ledipasvir, ribavirin and now asunaprevir to continue the fight.
Published in
Blogs
Tagged under
Monday, 29 February 2016 17:45
Hepatitis C Drugs may cost less than $100 to produce - Study by Dr. Andrew Hill
Even before Sovaldi, the first modern antiviral medicine for Hepatitis C, obtained FDA's market authorisation, a group of scientists from University of Liverpool correctly predicted the looming problem - Hepatitis C drugs will become one of the costliest and thus hardly accessible drugs on the planet.
Dr. Andrew Hill and his team from the Department of Pharmacology and Therapeutics, University of Liverpool, publish an article in prestigious Oxford Journals about how much it actually costs to manufacture modern Hepatitis C drugs on a large scale. The results might be very surprising, especially for people who were at the time paying $80,000 for 12-week treatment with Sovaldi.
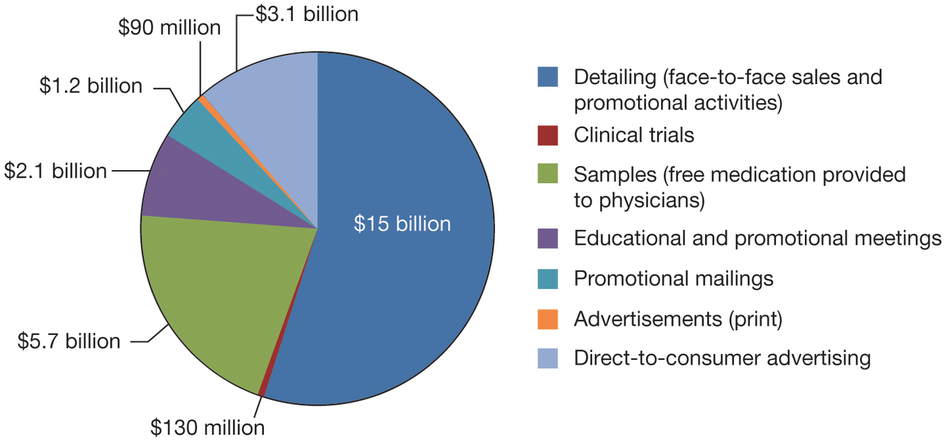
Breakdown of pharmaceutical company costs (2012) *Source: www.nature.com
In the case of Hepatitis C drugs, the production costs have no relation to the final cost of drugs like Sovaldi, Harvoni, Daklinza and Zepatier. Nonetheless, HIV drugs, which are very similar to Hepatitis C drugs, are being produced and sold at very low prices to more than 10 million of patients in third world countries. Dr. Andrew and his team tackled a question of how much would it cost to treat a Hepatitis C patient if we take into the account only the production costs.
The minimal cost for a therapy of Hepatitis C can be determined by determining the cost of production of these direct acting antivirals. Dr. Hill calculated how much does the production of sofosbuvir, daclatasvir and semiprevir regimen costs, based on how difficult it is to synthesize these direct acting antivirals and what is the dosage needed for a standard treatment.
What is more, the formulated cost was based on the premises that at least 1 million patients per year need to be treated. This means that the production of sofosbuvir and other molecules can be done in bulk and economy of scale can drive down the costs dramatically. For instance, pound per pound the production of lets say 10 tons of sofosbuvir is much more cost efficient than the production of 1 kg of sofosbuvir.
The Liverpool team also accounted for the margin that is needed to make pills out of synthesised DAA - in their models, this margin was 40% of the cost.
This brings the cost of sofosbuvir production for one treatment at $68-$136, or about $1 per pill. The current market price for a single 400 mg sofosbuvir molecule is about $1,000. Looking purely from this perspective, Hepatitis C treatment manufacturers like Gilead Sciences or AbbVie have a 100,000% margin on every pill they produce; and they produce millions of them every day.
This also explains why it is still financially reasonable for originator companies to approve the production of generic version of Hepatitis C drugs. Such treatment, primarily made by Indian pharmaceutical companies, cost about $1000. There is still a hefty 1,000% margin attached that is shared between the originator company (Gilead, AbbVie) and Indian pharmaceutical company actually making the pills.
Dr. Hill and his team at University of Liverpool determined that the production cost of daclatasvir for a 12-week treatment is only $10-30$. This is approximately how much a very good pizza costs; however, Bristol-Myers Squibb is pricing it beyond a price of a brand new Mercedes.
This might be a substantial cost for a production of an active molecule; however, it cannot compare with the actual price at which Janssen is marketing Olysio - $66,360.
Officials from companies that produce Hepatitis C drugs often argue that the high prices of Hepatitis C treatments are based on the potential costs on alternative treatments. In this case, alternative treatment for Hepatitis C patients is a liver transplant which costs about $250,000 in the US. Namely, the cost of production of sofosbuvir, daclatasvir and other DAA has nothing to do with price on the shelf. Even if the whole regimen would cost less than $1 to produce, the pharmaceutical companies would most probably still price the medications above $50,000.
This is what Dr. Andrew Hill had to say about the profits in Hepatitis C treatment area. "Gilead has already sold over $32 billion of two hepatitis C treatments in just over two years, with profit last year of $18 billion. The continued profit projections for the oral hepatitis C drugs are staggering, and stand in no relation to what it costs to make these drugs."
Dr. Andrew Hill and his team from the Department of Pharmacology and Therapeutics, University of Liverpool, publish an article in prestigious Oxford Journals about how much it actually costs to manufacture modern Hepatitis C drugs on a large scale. The results might be very surprising, especially for people who were at the time paying $80,000 for 12-week treatment with Sovaldi.
Production cost of Hepatitis C Drugs
When a pharmaceutical company discovers a novel drug, as was in the case of Sovaldi and company Gilead Sciences, the price that patients have to pay for it is substantially larger than the manufacturing cost for that drug. This is largely expected; a pharmaceutical company has to cover the costs of research and development, marketing, quality control and so on from the sales of the medicine.Breakdown of pharmaceutical company costs (2012) *Source: www.nature.com

In the case of Hepatitis C drugs, the production costs have no relation to the final cost of drugs like Sovaldi, Harvoni, Daklinza and Zepatier. Nonetheless, HIV drugs, which are very similar to Hepatitis C drugs, are being produced and sold at very low prices to more than 10 million of patients in third world countries. Dr. Andrew and his team tackled a question of how much would it cost to treat a Hepatitis C patient if we take into the account only the production costs.
Production cost of Sofosbuvir, Daclatasvir, Semiprevir - DAA (Direct Acting Antivirals)
The core of every drug is the API - active pharmaceutical ingredient. Hepatitis C is being treated with the use of 2 to 3 different APIs - we can also refer to them as direct acting antivirals (DAA). For example, the most basic modern Hepatitis C treatment includes Sovaldi with sofosbuvir as DAA and ribavirin which is by itself a DAA. Harvoni, for example, is a single-pill regimen that contains 2 DAA - sofosbuvir and ledipasvir.The minimal cost for a therapy of Hepatitis C can be determined by determining the cost of production of these direct acting antivirals. Dr. Hill calculated how much does the production of sofosbuvir, daclatasvir and semiprevir regimen costs, based on how difficult it is to synthesize these direct acting antivirals and what is the dosage needed for a standard treatment.
What is more, the formulated cost was based on the premises that at least 1 million patients per year need to be treated. This means that the production of sofosbuvir and other molecules can be done in bulk and economy of scale can drive down the costs dramatically. For instance, pound per pound the production of lets say 10 tons of sofosbuvir is much more cost efficient than the production of 1 kg of sofosbuvir.
The Liverpool team also accounted for the margin that is needed to make pills out of synthesised DAA - in their models, this margin was 40% of the cost.
Production cost of Sofosbuvir for standard 12-week treatment
The results are somewhat surprising; while Gilead charges $80,000 for a single treatment with sofosbuvir, the majority of direct acting antivirals production cost was calculated to be between $0.2 and $2.1 per gram. For 12-week treatment, taking 400 mg of sofosbuvir per day, a Hepatitis C patient will take a total of 33.6 grams of sofosbuvir during the course of his or her treatment.This brings the cost of sofosbuvir production for one treatment at $68-$136, or about $1 per pill. The current market price for a single 400 mg sofosbuvir molecule is about $1,000. Looking purely from this perspective, Hepatitis C treatment manufacturers like Gilead Sciences or AbbVie have a 100,000% margin on every pill they produce; and they produce millions of them every day.
This also explains why it is still financially reasonable for originator companies to approve the production of generic version of Hepatitis C drugs. Such treatment, primarily made by Indian pharmaceutical companies, cost about $1000. There is still a hefty 1,000% margin attached that is shared between the originator company (Gilead, AbbVie) and Indian pharmaceutical company actually making the pills.
Production cost of Daclatasvir for standard 12-week treatment
Daclatasvir is a key ingredient in Daklinza, produced by Bristol-Myers Squibb. Treatment of Hepatitis C with Daklinza in the US costs a total of $63,000. But how much does the production of daclatasvir really costs?Dr. Hill and his team at University of Liverpool determined that the production cost of daclatasvir for a 12-week treatment is only $10-30$. This is approximately how much a very good pizza costs; however, Bristol-Myers Squibb is pricing it beyond a price of a brand new Mercedes.
Production cost of Semiprevir for standard 12-week treatment
Semiprevir is a direct acting antiviral in Janssen's Olysio. The production cost of the semiprevir was found to be $130–$270 for the standard treatment.This might be a substantial cost for a production of an active molecule; however, it cannot compare with the actual price at which Janssen is marketing Olysio - $66,360.
Why are Hepatitis C patients paying over $50,000 for treatments that can be produced for less than $100?
In short, it is how the current pharmaceutical industry operated. Pharmaceutical companies have much flexibility as far as pricing is concerned; especially the originators where their drugs are basically the only such drugs available - it is an effective monopoly.Officials from companies that produce Hepatitis C drugs often argue that the high prices of Hepatitis C treatments are based on the potential costs on alternative treatments. In this case, alternative treatment for Hepatitis C patients is a liver transplant which costs about $250,000 in the US. Namely, the cost of production of sofosbuvir, daclatasvir and other DAA has nothing to do with price on the shelf. Even if the whole regimen would cost less than $1 to produce, the pharmaceutical companies would most probably still price the medications above $50,000.
'$100-$250 per Hepatitis C treatment is feasible'
Dr. Hill and his team predict that in 15 years the prices of Hepatitis C production might come down because of the large-scale manufacturing. In theory, this could make Hepatitis C treatment available to patients in low-income countries. However, it is important to understand that the final pricing of a product is not primarily determined by its costs of production, but by interests of pharmaceutical companies for profit and the willingness of patients to succumb to higher prices since their lives are at stake.Turn to FixHepC Buyers Club
A good news is that large-scale manufacturing is already bringing the cost of Hepatitis C direct acting antivirals down. The production costs are not as low as $100-$250 yet but with the courtesy of Dr. Freeman of FixHepC Buyers Club, you can avoid paying tens of thousands of dollars for the medications. The Buyers Club provides you with the direct acting antiviral drugs equally capable of curing Hepatitis C as Sovaldi, Harvoni, Olysio, Daklinza and so on, for a fraction of the cost. You can check the prices of sofosbuvir, daclatasvir, ledipasvir here.This is what Dr. Andrew Hill had to say about the profits in Hepatitis C treatment area. "Gilead has already sold over $32 billion of two hepatitis C treatments in just over two years, with profit last year of $18 billion. The continued profit projections for the oral hepatitis C drugs are staggering, and stand in no relation to what it costs to make these drugs."
Published in
Blogs
Friday, 26 February 2016 20:50
Hep C treatment discount for US Army Veterans? Not even Bernie Sanders can stop Gilead's extreme pricing
Here are some quick facts about Hepatitis C and US Army Veterans.

The Chairman of HVAC, Florida Republican Rep. Jeff Miller, who oversees where the budget for US Army Veterans goes, is outraged by the extreme pricing that is being used by Gilead. He went so far as to accuse the produced of price-gouging and 'picking and choosing' it's pricing models based on the target customers and location. For example, Gilead has made Sovaldi, a Hepatitis C drug, available for $900 in Egypt and 90 other countries around the world - at home, in the US, however, the endangered US Army Veterans have to pay $40,000 for exactly the same medications. In Miller's opinion, this is flat out considered an opportunism and an extortion of Army Veterans.
Sanders invited Gilead executives to attend the hearing but was declined; as it happens, Gilead executives cited overseas business travel as an excuse not to attend the hearing.
"With numbers like these, we're not talking about a company looking to make ends meet, or even fund their next great medical breakthrough,” Sanders said. "We're looking at a company who is milking a cash cow for everything it's worth."

Pharmaceutical companies generally justify high costs of original medications because of the costs that went into research and development, and expected costs of R&D for the new products. In short, the incentive to innovate is driven by the funds raised of selling the previous drugs. Arguably, this is not the case in Hepatitis C and Gilead - Sanders stating they are milking Hepatitis C patients, US Army Veterans among them, for money that translates directly into corporate profits is quite on point.
This is a very large amount gifted by the Congress but how far can it go? Considering that VA is paying the minimal cost for Hepatitis C treatment - Sovaldi for $40,000 - the number of veterans that can be treated with this budget in 2016 is 37,500. That may seem a lot but what if you're one of more than 162,500 veterans who won't get the treatment in 2016? Wait for another year in hopes you will get it while Hepatitis C virus is destroying your liver?
In the end, we leave you with the quote by Chairman of Veterans Affairs Committee:
“Government shouldn’t be in the business of telling private companies what to charge their customers,” Miller wrote. “[But] Gilead's price discrimination against American veterans and the organization established to care for them is a slap in the face to the millions who depend on VA health care as well as the taxpayers who generously fund the department.”
- Since 2013, we have a cure for Hepatitis C that is about 95% successful
- In the US, more than 200,000 Army Veterans suffer from Hepatitis C
- $40,000 for Sovaldi (400mg sofosbuvir)
- $68,000 for Harvoni (90mg ledipasvir/400mg sofosbuvir)
'Hepatitis C drugs price-gouging' - Bernie Sanders
The issue of extreme pricing of the novel Hepatitis C medications such as Gilead's Sovaldi and Harvoni is being raised time and time again. In Europe, the national health care systems are being challenged and endangered because of the billions that would be required to pay for treatment of Hepatitis C patients. In the US, the situation is even worse - on the home ground, the US-based pharmaceutical giant Gilead Sciences, producer of Hepatitis C medications, is charging up to $94,500 for a single Hepatitis C treatment. Safe to say, one of the most Hepatitis C endangered populations are the US Army Veterans and they are being appalled by the astonishing prices Gilead is demanding for the medications that can be produced for less than $1,000.House Veterans' Affairs Committee on Hepatitis C

The Chairman of HVAC, Florida Republican Rep. Jeff Miller, who oversees where the budget for US Army Veterans goes, is outraged by the extreme pricing that is being used by Gilead. He went so far as to accuse the produced of price-gouging and 'picking and choosing' it's pricing models based on the target customers and location. For example, Gilead has made Sovaldi, a Hepatitis C drug, available for $900 in Egypt and 90 other countries around the world - at home, in the US, however, the endangered US Army Veterans have to pay $40,000 for exactly the same medications. In Miller's opinion, this is flat out considered an opportunism and an extortion of Army Veterans.
Bernie Sanders is taking on Gilead for Hepatitis C pricing
As early as in late 2014, Bernie Sanders, a current Democratic nominee for 2016 US presidency and, summoned a hearing about the practices of extremely high pricing in Hepatitis C treatment area. He is reported to be especially concerned with the heavy toll the costs of Sovaldi and Harvoni treatments have on Veteran Affairs annual budget.Sanders invited Gilead executives to attend the hearing but was declined; as it happens, Gilead executives cited overseas business travel as an excuse not to attend the hearing.
"With numbers like these, we're not talking about a company looking to make ends meet, or even fund their next great medical breakthrough,” Sanders said. "We're looking at a company who is milking a cash cow for everything it's worth."

Pharmaceutical companies generally justify high costs of original medications because of the costs that went into research and development, and expected costs of R&D for the new products. In short, the incentive to innovate is driven by the funds raised of selling the previous drugs. Arguably, this is not the case in Hepatitis C and Gilead - Sanders stating they are milking Hepatitis C patients, US Army Veterans among them, for money that translates directly into corporate profits is quite on point.
How much do US Army Veterans pay for Sovaldi and Harvoni?
Sovaldi, the first modern Hepatitis C treatment was introduced in late 2013, and in 2014 the Veterans Health Administration helped to treat more than 5,400 Army Veterans. The total cost of these treatments was $370 million which is approximately $68,500 per veteran. Needless to say, that money could go a very long way if used for other benefits veterans might get. Paying for life-saving medicines for Army Veterans is a no-brainer but the reality of Hepatitis C treatment is its high cost. Will veterans see less of other benefits because a big amount of Veterans Affairs budget has to go to Gilead for Hepatitis C drugs?Good (and bad) News for Veterans in 2016
Because of more than 200,000 veterans suffering from Hepatitis C and the high costs of treatment, the VA requested for additional funds for the 2016 fiscal year. Congress approved that $1.5 billion in total should go for treating US Army Veterans with Hepatitis C.This is a very large amount gifted by the Congress but how far can it go? Considering that VA is paying the minimal cost for Hepatitis C treatment - Sovaldi for $40,000 - the number of veterans that can be treated with this budget in 2016 is 37,500. That may seem a lot but what if you're one of more than 162,500 veterans who won't get the treatment in 2016? Wait for another year in hopes you will get it while Hepatitis C virus is destroying your liver?
Turn to FixHepC Buyers Club
As always, FixHepC Buyers Club welcomes all Hepatitis C patient in search of low-cost Hepatitis C medications. We provide you with everything Hepatitis C patients need to get well for less than $2000 under a watchful eye of Dr. Freeman. Contact us for more information.In the end, we leave you with the quote by Chairman of Veterans Affairs Committee:
“Government shouldn’t be in the business of telling private companies what to charge their customers,” Miller wrote. “[But] Gilead's price discrimination against American veterans and the organization established to care for them is a slap in the face to the millions who depend on VA health care as well as the taxpayers who generously fund the department.”
Published in
Blogs
Tagged under
