Super User
Sofosbuvir+Daclatasvir results shine - bad news for Epclusa and Maviret?
The combination of Sovaldi (Sofosbuvir) and Daklinza (Daclatasvir) was the world's first pan-genotypic Hepatitis C treatment. In the USA and Europe the $140,000 price tag of this combination means it has seen relatively little use, but in the world of generics it has seen extensive use because Daclatasvir is much cheaper to produce than either Ledipasvir (in Harvoni) or Velpatasvir (in Epclusa) so it provides the least expensive treatment option.
Ok, so it's more affordable, but is it as good as either Epclusa or Maviret? These are the new kids on the block, they are more expensive, so surely they are newer and must be better? Not really...
Abbvie conducted a trial called ENDURANCE-3 which demonstrated that Maviret - Glecaprevir + Pibrentasvir - (G/P) is "non-inferior" to the combination of Sofosbuvir + Daclatasvir (SOF/DCV). What is "non-inferior"? Well SOF/DCV achieved 97% SVR and G/P achieved 95% SVR12 in the difficult to treat GT3 population. Maviret is "non-inferior" is one way of phrasing it. SOF/DCV is "slightly superior" would be another way although this is not statistically significant.
Our REDEMPTION trials put the results for generic SOF/DCV in the range 93% (for GT3) to 100% (for GT2, GT5 and GT6) with 97% in GT1 and 95% in GT4 and are based on the results of over 1000 patients.
Published two days ago we have Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt - where the overall results were 95.1%
So there you have it. We have 3 pan-genotypic options. They are all good, and all hit ~95%+ cure. Two are newer, more expensive, and have far less real-world proof. Strip off the marketing hype and if you were looking towards mass scale treatment SOF/DCV looks like the go-to medication because you can get the same cure rates for less cost, at least when using generics.
What happens when you finish your Hep C treatment?
Recently I have been getting a number of enquires from patients who have just finished their tablets, all asking the same questions, so here is a quick primer. If you are looking for this sort of information you will find a lot more detail in our Frequently Asked Questions and on the Forum.
On treatment 99.5% of patients will drop to <15 on their HCV RNA. This is nice but does not prove cure. We need virtually zero viruses (less than 100 in total) to remain in your system to achieve cure.
When the tablets finish your body starts to remove the medication from your system. Within 24 hours there is less than 1/2, 48 hours 1/4, 3 days 1/8, 4 days 1/16, 5 days 1/32, 6 days 1/64, 7 days 1/128 and by this stage there is definitely not enough of the drugs left in your system to kill the virus.
So with the medicines now below effective levels one of two things happens.
- The virus starts to grow back because there is still some left, or...
- Nothing - ie the virus does not grow back because there is no virus left to grow back
If the virus has not grown back 4 weeks after the last tablet was taken and the HCV RNA PCR remains <15 then there is a 97% chance this will be permanent. We call this Sustained Virological Response (SVR) and because this one happened at 4 weeks we call it SVR4
If the virus has not grown back 12 weeks after the last tablet was taken and the HCV RNA PCR remains <15 then there is a 99.7% chance this will be permanent. We call this Sustained Virological Response (SVR) and because this one happened at 12 weeks we call it SVR12
If the virus has not grown back 24 weeks after the last tablet was taken and the HCV RNA PCR remains <15 then there is a 100% chance this will be permanent. We call this SVR24 but a better word is cure.
Please note this. Your Hepatitis C Antibody (HCV Ab) test will remain positive. Your body produces antibodies in response to infection and these persist for years. For example, if you have Hepatitis B vaccination you will have antibodies that persist for years, and often for life. Similarly to Hepatitis B without an exposure event (booster dose for Hep B, ongoing infection for Hep C) the levels of antibody will slowly fall over time, and may (after 5-10 years) fall below the level we use as a cut off, however, you should expect to remain antibody positive for many years. Cure is a negative HCV PCR RNA and the antibody remains as a marker of past infection.
This period of waiting can be stressful. Forums like https://fixhepc.com/forum can provide useful support.
With the SVR tests liver functions are normally conducted at the same time. These liver function results come back the next day, rather than a week later. If your liver functions remain similar to what they were on treatment (+/- 20%) you can be confident you have not relapsed. I have treated about 3000 patients and, as the statistics predict, seen over 100 relapses. In every case, the patient has been aware of the relapse before the results came back.
If you feel well you almost certainly are, but if you feel unwell it is worth noting that as well as over 100 relapses I have seen about 100 patients assigning every little twinge in their body to relapse (and they did not) so don't panic if you feel anxious - it's pretty normal. The result will be what it will be and worrying is about as effective as chewing bubble gum while trying to solve an algebra equation. If you do relapse it's possible to have another try and the results of take 2 are good although the costs of treatment do double.
It is normal to have some twinges from your liver. It's been suffering for years and now has the chance to recover. Some patients notice this.
In terms of getting pregnant, you should wait until 6 months after the tablets have finished. Ribavirin, in particular, is known to cause birth defects so you need to leave enough time for it to exit your system.
Finally, if you liver functions are not back to normal after treatment this should be looked into. A small percentage of patients with Hepatitis C also have another liver disease and we (as in doctors) have been assuming it is all due to the Hep C... Here is a list of things to look for if your LFTs do not normalise https://www.mayoclinic.org/symptoms/elevated-liver-enzymes/basics/definition/sym-20050830
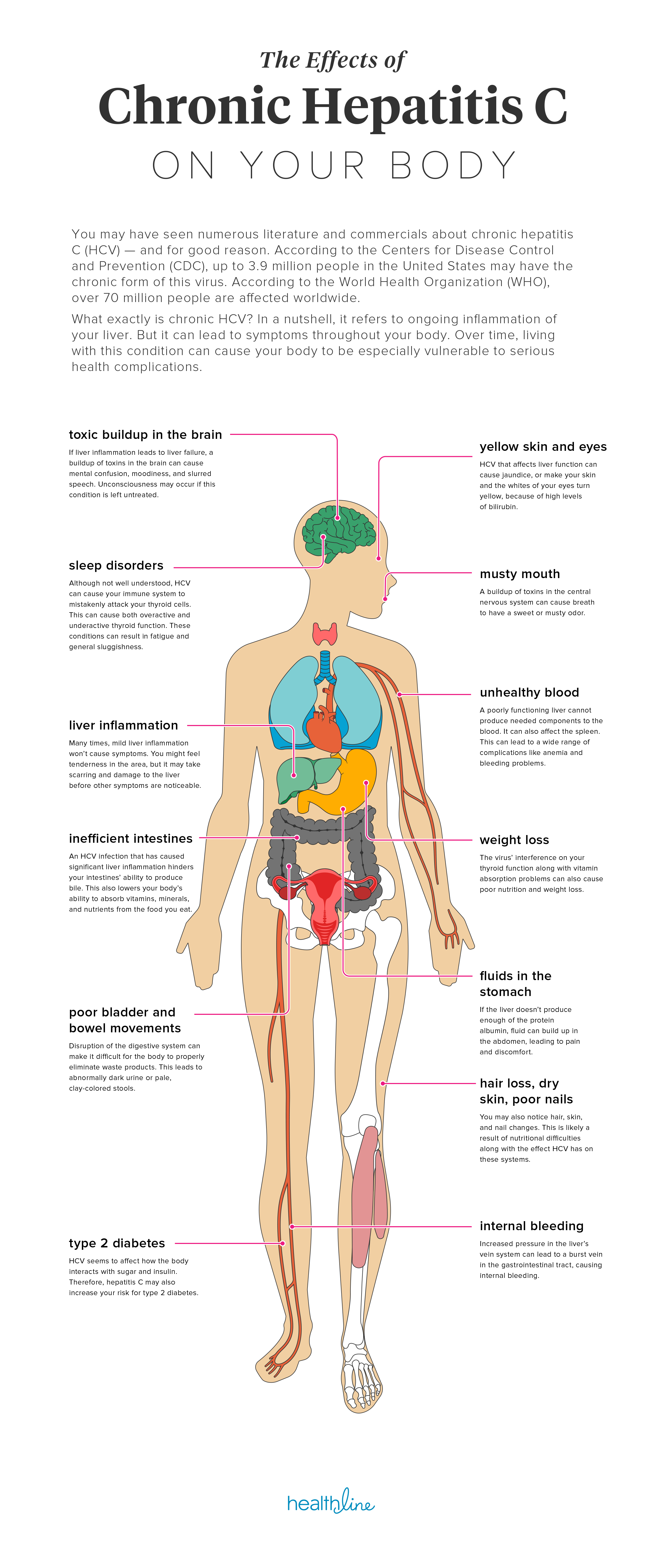
71% decrease in cancer rate for Hep C patients with SVR
Some time ago we saw some very bad science published. This suggested that treating Hepatitis C confers no benefits. While experts around the world expressed their firm view this was complete and utter rubbish here is something simple to understand. Get treated, get SVR and... you are far less likely to die of liver cancer.
This slide came from a presentation on Clinical Care Options entitled "Current HCV Therapy". The full slides are attached here:
This is just one of the reason treating your Hepatitis C sooner rather than later is a good idea. Push for coverage and appeal, but if you can't get access we know with 100% certainty that generic HCV medications deliver the same results - only the price is different (they are affordable).
Pharmaniaga, Pharco and DNDi Sign Agreement to Provide Affordable Hepatitis C Treatment in Malaysia
KUALA LUMPUR/GENEVA – 13th November 2017 – Malaysian pharmaceutical company Pharmaniaga Logistics Sdn Bhd (Pharmaniaga), Egyptian pharmaceutical company Pharco Pharmaceuticals (Pharco) and non-profit research and development organization Drugs for Neglected Diseases initiative (DNDi) have signed a collaboration agreement to supply a new hepatitis C treatment regimen to be sold for US$300 in the public sector in Malaysia.
“It is estimated that there are around 450,000 people living with hepatitis C in Malaysia but most of them have been unable to access effective new hepatitis C treatment because of its very high price,” said Dato’ Farshila Emran, Managing Director of Pharmaniaga. “This agreement enables access to cheaper alternatives for patients in Malaysia, and we are proud to be embarking on this collaboration with DNDi and Pharco that would not have been possible without the Ministry of Health’s support.”
In partnership with the Malaysian Ministry of Health, DNDi is currently running clinical trials testing a potentially pan-genotypic treatment, combining the drug candidate ravidasvir, produced by Egyptian drug manufacturer Pharco Pharmaceuticals, with the existing hepatitis C medicine sofosbuvir. The clinical trial is ongoing in six hospitals and is co-sponsored by the Malaysian Ministry of Health, with initial results expected in early 2018.
“We applaud the Malaysian government in its efforts and commitment to make available affordable access to safe and effective treatments for hepatitis C,” said Jean-Michel Piedagnel, Head of DNDi South-East Asia. “The strategic investment from the Ministry of Health in helping develop a public health approach to the epidemic is remarkable.”
The agreement covers the supply of ravidasvir, once approved in Malaysia, and supply of sofosbuvir. A Government Use licence issued by the Malaysian Ministry of Domestic Trade, Co-operatives and Consumerism in September 2017 enables the importation of generic sofosbuvir in order to make this drug available in the public health system throughout the country at affordable prices.
“We hope that our collaboration with Pharmaniaga and DNDi to develop a treatment regimen that costs less than US$1 per day will lead to widespread access to safe, effective, and affordable treatment for hepatitis C patients in Malaysia and around the region,” said Dr. Sherine Helmy, CEO of Pharco Pharmaceuticals.
Currently a full 12-week course of treatment is available in Malaysia for around US$70,000. With the combination of generic sofosbuvir and ravidasvir, the cost for the same treatment will be made affordable with an almost 100% decrease in price.
We have the power to FixHepC, but the real question is...
We have the power to FixHepC, but the real question is... Do we have the willpower?
This was how Dr James Freeman ended his presentation at the World Hepatitis Summit in Brazil.
You can watch the full presentation on YouTube.
In essence, if we applied the same effort to HCV as we do to HIV this epidemic could be over within a single year. Currently, we treat 18 million patients with one year's supply of multiple antiviral drugs. That would translate to 72 million 3 month treatment courses - enough to treat every man woman and child with HCV.
We still have the enormous challenge of finding those patients in time, but we clearly have the funding capacity and the industrial capacity to get it done.
One year, that's all it would take.
The slides are attached below:
Slides from Dr James Freeman's Generics Presentation at the World Hepatitis Summit 2017
Qualifying for Social Security Disability Benefits With Hepatitis C
Hepatitis C is a disease that can make you feel old before your time. While there is no doubt that treatment with the new generation of direct acting antivirals like Harvoni is by far the best option this is not available to everyone, either because they don't qualify under their insurance, don't have insurance, or can not afford the $1250 cost to get generic hepatitis c treatment.
This guest blog was written by the Outreach Team at Disability Benefits Help and outlines how you can get help if you have become too sick to work. This includes both financial support and the potential to access to subsidised treatment.
Qualifying for Social Security Disability Benefits With Hepatitis C
If you have been diagnosed with Hepatitis C and will be unable to work for at least one year, you might be eligible for Social Security disability benefits. The Social Security Administration (SSA) offers financial resources for people who are unable to work for an extended period of time due to a serious illness. Hepatitis C will not automatically qualify, and unfortunately, most people with the condition will not be approved. Severe cases will qualify though, meaning you could be eligible for payments to help with your daily living expenses, as well as access to health insurance to pay for your hepatitis treatments.
12 Months and Social Security Disability
Social Security disability benefits are never temporary. They’re only available for people who expect to have a disability that lasts at least a year. This means that if you’ve recently been diagnosed with hepatitis C and have a good prognosis with minimal liver damage and a strong treatment plan, you likely won’t qualify. You also will not qualify if you’ve had hepatitis C for years but your symptoms are managed well. The SSA will not consider you to be “disabled” unless you are completely unable to earn at least $1,170 per month (in 2017). If you expect to be finished with treatments within a few months, or your condition is well managed, it’s not advisable to apply for disability benefits.
Qualifying Via the Blue Book
For those with severe liver damage, medical qualification is possible and potentially guaranteed, depending on your liver function. The SSA evaluates all hepatitis C applicants under its own medical guide, known as the Blue Book. The Blue Book is a manual listing all potential disabling conditions, plus the symptoms you’d need to qualify. Hepatitis c would fall under Section 5.05: Chronic liver disease. Chronic liver disease is arguably the most complicated listing in the Blue Book. There are actually seven ways to qualify under this listing, and all require medical tests evaluating your serum levels, blood quality, and more. The Blue Book was written for medical professionals and is available entirely online, so please review the listing with your hepatologist! He or she can help you navigate the guide and determine if your hepatitis c meets any liver disease listings. If you require a liver transplant, rest assured that your liver disease will qualify. You will also automatically qualify for disability benefits for at least 12 months after a liver transplant surgery.
Starting Your Application
Most people can apply for Social Security disability benefits online. If you’d prefer, you can also apply in person at your closest office by scheduling an appointment at 1-800-772-1213. There are over 1,000 offices across the US.
You’ll usually hear back from the SSA within three to five months. If your application is denied, do not give up! The SSA has a thorough appeals process, which allows you to fight your claim. While just 30% of applicants are initially applied, nearly 70% are eventually approved after pursuing their claims further.
This article was written by the Outreach Team at Disability Benefits Help. They provide information about disability benefits and the application process. To learn more, please visit their website at www.disabilitybenefitscenter.org or by contacting them at This email address is being protected from spambots. You need JavaScript enabled to view it..
If you have Hepatitis C where can you get free confidential support?
About 1 in 100 people, from all walks of life, have Hepatitis C so it is pretty common. Because it is a highly stigmatised disease, linked in the public mind to IV drug use, most patients choose to keep their diagnosis confidential. Despite the fact that the evidence shows most patients were infected too young to have been involved in IVDU, and many patients got it from blood transfusions, tattoos, and unsafe medical procedures, changing the stigma is probably an impossible task.
What this means is that having Hepatitis C is a very lonely experience for many people. Having to deal with the symptoms is one thing, but having to do it alone just adds to the burden. The good news is that help is out there!
One of the great things about the Internet is it allows people from across the globe to connect with other people with a common interest and with complete anonymity.
To find people like you, who have walked in your shoes and know how it feels, please visit the FixHepC forum - https://fixhepc.com/forum
You can browse the forum without logging in but to post you do need to create a login. Most people just use an anonymous email address to do that so there is no problem about maintaining your annonymity.
There are now highly effective treatments available with minimal side effects. For some patients, insurance and Medicaid can come to the rescue. For others, it might be a patient assistance program or the parallel importation of generics (these are the same medications but with a $1000 price tag for the whole treatment rather than a single pill). It is a cruel and unusual punishment to know that cure exists, but not for you.
Drop in to the FixHepC forum and find out how people just like you have navigated the varying processes that can see you get supported, treated and cured.
AASLD 2017: Generics shown to be bioequivalent to originator brands
Just out of embargo for AASLD 2017 is the rather innocuous sounding Abstract 1078 which says, in brief, that these generic DAAs are inarguably proven the same as the originator DAAs.
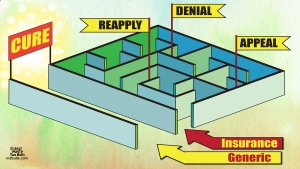
Bioequivalent pharmacokinetics for generic and originator Hepatitis C Direct Acting Antivirals
Andrew M. Hill1, Loai Tahat2, Mohammed Khalil Mohammed3, Sanjay Nath4, Rabab Fayez Tayyem3, James A. Freeman5, Ismahane Benbitour7, Sherine Helmy6; 1Department of Translational Medicine, University of Liverpool, Liverpool, United Kingdom; 2Pharmaceutical Research Unit, Amman, Jordan; 3ACDIMA BioCentre, Amman, Jordan; 4Faculty of Medicine, Imperial College London, London, United Kingdom; 5GP2U Telehealth, Hobart, TAS, Australia; 6R&D Project Innovations, Pharco, Cairo, Egypt; 7BEKER Laboratories, Algiers, Algeria
Background: Mass production of low-cost generic directacting antivirals (DAAs) will be required to achieve targets of eliminating hepatitis C (HCV) by 2030. Gilead and Bristol-Myers Squibb have granted voluntary licenses to generic companies to mass-produce the DAAs sofosbuvir and daclatasvir at low cost. However generic manufacturers need to demonstrate bioequivalent pharmacokinetics for their generic DAAs compared to the originator versions, to fulfil World Health Organization standards for pre-qualification.
Methods: Randomised, single-dose, two-way, two-period pharmacokinetic studies were performed in 35-54 healthy volunteers, to compare generic forms of sofosbuvir and daclatasvir with the originator versions. Generics evaluated were from Pharco (Egypt), Beker (Algeria) and Hetero (India), versus originator sofosbuvir (Gilead) and daclatasvir (Bristol-Myers Squibb). All studies were conducted under Good Clinical Practice (GCP). Plasma concentrations of each DAA were assessed over 24 hours. Maximum concentration (Cmax) and Area Under the Curve (AUC) were calculated for each subject. Geometric mean ratios and associated 90% confidence intervals were used to compare each generic DAA with the originator version. Pre-specified limits for the 90% confidence intervals were 80% to 125% of the originator pharmacokinetic concentrations for AUC, and 69-145% for Cmax.
Results: Table 1 shows summary Geometric mean ratios for generic versus originator versions of sofosbuvir and daclatasvir. NB Results from Natco and Virchow came in after the deadline for AASLD submission, but are included here.
Table 1: Geometric mean ratio (90% confidence intervals) for generic versus originator HCV DAAs
| Drug | Company | N | Cmax | AUC0-∞ |
| Sofosbuvir | Pharco | 36 | 101.0 (88.1-115.7) | 103.5 (97.6-109.7) |
| Daclatasvir | Pharco | 36 | 106.9 (100.2- 114.0) |
103.7 (98.3-109.4) |
| Sofosbuvir | Beker | 35 | 95.4 (84.7-107.5) | 98.5 (91.6-106.0) |
| Daclatasvir | Beker | 35 | 35 104.1 (93.1-116.3) | 103.0 (94.4-112.4) |
| Sofosbuvir | Hetero | 54 | 95.7 (97.2- 105.2) | 100.8 (96.2- 105.6) |
| Sofosbuvir | Natco | N/A | 96.1 (81.0-114.0) | 100.7 (94.2-107.8) |
| Daclatasvir | Natco | N/A | 94.5 (83.1-107.4) | 96.5 (87.1-106.8) |
| Sofosbuvir | Virchow | 24 | 94.8 (83.3-107.9) | 95.8 (86.9-105.7) |
All three (now expanded to 5) generic forms of sofosbuvir met pre-specified bioequivalence criteria. The Cmax and AUC of daclatasvir were bioequivalent in both cases. There were no serious adverse events observed.
Conclusions: The pharmacokinetics of generic sofosbuvir and daclatasvir were bioequivalent to the originator versions, in three independent single-dose PK studies. WHO pre-qualification of bioequivalent generic DAAs could then permit their export to high-burden countries for mass treatment programmes.
Disclosures: James A. Freeman - Advisory Committees or Review Panels: Pharco Pharmaceuticals, Beker Pharmaceuticals, Beacon Pharmaceuticals; Stock Shareholder: FixHepC, GP2U Telehealth
Ismahane Benbitour - Management Position: BEKER Laboratories
Sherine Helmy - Board Membership: Presidio Pharmaceuticals; Stock Shareholder: Pharco Pharmaceuticals
The following people have nothing to disclose: Andrew M. Hill, Sanjay Nath
Bye bye limitatio (and generics) - Open access to the new Hepatitis C medications for all Swiss patients
Switzerland has just announced that all limitations on access to the new DAAs will be abolished allowing treatment for all. Great news for Swiss patients. Here is an article from the Swiss Hepatitis C Association about it. If you are not lucky enough to live in Switzerland generic versions of the new medications are available for 1% of the originator prices. It's interesting to see that all the countries that have parallel imported generics at scale have managed to strike great pricing deals that allow things like this to happen. Australia, Canada, New Zealand, Italy, Switzerland... The list is growing.
Bye bye limitatio – People with hepatitis C are relieved
The rationing of hepatitis C drugs laid down by the Federal Office of Public Health (FOPH) about three years ago will be abolished as of 1 October 2017. For the first time, all patients can be treated regardless of the type of virus and disease progression.
The rationing of Gilead’s hepatitis C medicines will also be discontinued on October 1,2017. Harvoni and Epclusa can thus be prescribed to all hepatitis C patients for the first time, regardless of liver damage. This was after the rationing had already been abolished for Zepatier on July 1 and for Viekirax/Exviera on August 1 for all those affected with genotypes 1 or 4.
The abolition of rationing was accompanied by substantial price reductions, which are now also expected for Gilead products. Since Gilead’s Epclusa can be used for all 6 genotypes, all hepatitis C patients, regardless of their stage of development, can be treated and cured with a very high probability from October 1. This is excellent news for affected patients and represents an important step towards the elimination of hepatitis C in Switzerland.
This concludes a sad chapter in the history of the Swiss healthcare system. For years, leading representatives of the FOPH have claimed that it makes no sense to treat “healthy people”. As a result, thousands of people with hepatitis C were left untreated and continued to suffer from the symptoms of their disease for years. We welcome the latest developments and are extremely pleased with the change of heart in the FOPH. With the abolition of rationing, the core requirement of the SHCA patient organisation and the Positive Council, the “treatment of all HCV patients”, has finally been fulfilled.
This is the demand we have made since our petition to Federal Councillor Berset in July 2015.
The FOPH will boast of having achieved price reductions. This confirms our accusation that the Federal Office fought a price war on the back of the patients for years. This is ethically untenable and we warn against pursuing the same strategy for other life-threatening diseases. Under no circumstances should those affected be the victims of such price wars. We demand that our Parliament develops the necessary instruments to enable the Federal Office to conduct future price negotiations more constructively and transparently.
We would like to thank the experts of the Swiss Hepatitis Strategy, who have worked tirelessly with us to promote the well-being of those affected, and we regard the case of the limitation as a joint success for civil society.
We would also like to thank the industry, whose medicines have cured us and hopefully will now enable many other hepatitis C patients to lead a new life. Their willingness to compromise in the negotiations with the FOPH made it possible to abolish rationing.
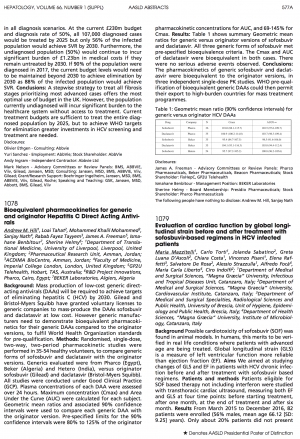
The Effects of Hepatitis C on Your Body
What exactly is chronic HCV? In a nutshell, it refers to ongoing inflammation of your liver. But it can lead to symptoms throughout your body. Over time, living with this condition can cause your body to be especially vulnerable to serious health complications. Here is a great infographic from Healthline about the effects of Hepatitis C on your body.