Super User
You Can't Patent The Sun
In 1961, Polio Vaccine Inventor Jonas Salk explained his decision to make his invention freely available with the words "You can't patent the sun".
185,000,000 people are infected with Hepatitis C and since a cure was invented only 613,000 people have been treated - during the same time 1,500,000 have died.
To mark World AIDS day on 1st December we have made this short video. If you care please share.....
You Don't Look Like A Junkie
You Don't Look Like A Junkie from Kate Brown on Vimeo.
Pharma Scores Rap Drug Cover-Ups
This is a screenshot of an article in the Australian today.
Here is a link to the full text of the study published in the British Medical Journal
http://bmjopen.bmj.com/content/5/11/e009758.full.pdf
And topping the list is..... No prizes for guessing.
The Real Development Cost of Sofosbuvir
So Big Pharma's argument about pricing goes like this:
We spend a lot on R&D so we need to make a lot back when we find a winner.
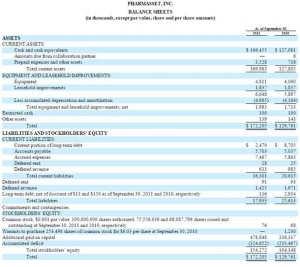
Now with Pharmasset we can look at the balance sheet immediately prior to Gilead's purchase. At the time the sunk capital cost (accumulated deficit) was $324 million. That's a lot of money, but nothing like the $7 billion valuation in the market (20x) or the sale price of $11 billion (33x).
That $324 million represents almost the ENTIRE development cost of this medication. There would have been another $50 million for the Phase 3 trials. Call it $400 million all in.
With some 613,000 people treated to date at around $50,000 per person the return is already north of $30 billion. That is 60 x the sunk cost in 3 years.
To treat the entire US population would be north of $200 billion. That is 500 x the sunk cost.
I believe Big Pharma takes big risks and has a right to big profits. The investors in Pharmasset got a 20 x + return and took almost all the risk. That does not seem entirely unfair. Gilead on the other hand took very little risk and is already sitting on 3 x at the price of 185,000,000 of our fellow citizens of the world being unable to access treatment and 500,000 a year perishing of a treatable disease.
If we are happy to live in a world where the cure for cancer is invented but only the super rich can afford it then that's fine.
If however we want to live in a world where patient lives balance patent rights we probably need to start thinking about how we provision decent, but not indecent profits and pricing practices.
Houston, I think we have a problem....
You've almost certainly seen a version of this picture. It's called earthrise and really hammers home the idea that we live co-operatively on a tiny speck in a dark black void.
This particular version was taken out of the window of Apollo 13 on its ill fated journey to the moon.
So, why is it here?
According to Reuters John Martin, CEO of Gilead Sciences, has taken home $400.6 million dollars between 2009-2013 and is on track to take home $600 million making him the best remunerated executive in the USA.
Since HCV DAA medications became available 500,000 people have been treated, 1,500,000 people have died and 150,000,000 remain infected.
At that rate, this week one man will take home over $2 million based on profits from HCV medications while 10,000 fellow citizens of the world will die due to lack of access to those medications.
I don't know about you, but to me, there is something not quite right about these numbers.
I wonder what sort of world we would be living in if the companies behind penicillin and polio vaccine had behaved in the same way...
Heard It Through The Grapevine
Rumour has it that PBS listing of HCV DAAs is not going to happen this year. It's not official yet, but...
We've started treatment for 5 patients on the transplant list in the last week, all referred from various centers.
If you're F4 waiting might not be your best option.
It is not just business, it's personal - I have failed, but I will do better
I just sent an email called "There are no words" because... there simply are not.
Dear L,
Please accept my condolences about M's passing and know that the opportunity to meet such a beautiful human being, who became so suddenly unwell, and still, staring death in the eye was weighing up the financial needs of his family with the costs of personal survival touched me.
It helped turned abstract statistics into the thought: "f**k, this is real, people die from this, and I need to help."
I am sorry we were unable to get medications to M in time or at all.
I have copied in Nevil, who was my personal patient zero, challenged me to become involved, and made the connection.
Without both M and Nevil I might have chosen the coward's route and satisfied my conscience with an "It's all too hard" cop out.
I wish more could have been done but it would be fair to say I helped setup FixHepC as a direct result of meeting both Nevil and M.
2 million people from 100 countries have found it.
Last week I started over 50 patients on treatment, including 5 on the transplant list.
None are M but all have family and friends.
There are no words, but I share this with my patients when faced with similar circumstances:
http://psychcentral.com/lib/the-5-stages-of-loss-and-grief/
For some it provides, if not solace, at least understanding of what you must be feeling and comfort that it is normal.
My thoughts are with you.
M's loss will not be in vain.
Kind Regards
James
Bangladesh Is Open For Business
We have previously tested Incepta's Twinvir and today have received samples of Beacon Pharma's Sofosbuvir, Daclatasvir and Ribavirin products for testing.
We will share the testing results as soon as they are available.
The quality of production of the packaging is extremely impressive, and I must say somewhat better than Sofosbuvir and Ribavirin I have seen from India.
Great packaging won't cure anybody, but it is very encouraging to see such meticulous attention to detail.
Beacon's product even have holographic seals.
Who Are We And Where Do We Come From?
Here is some interesting data from the Google Analytics about the FixHepC visitor demographics.
It's kind of spooky that Google knows your age and sex, but here's their analysis.
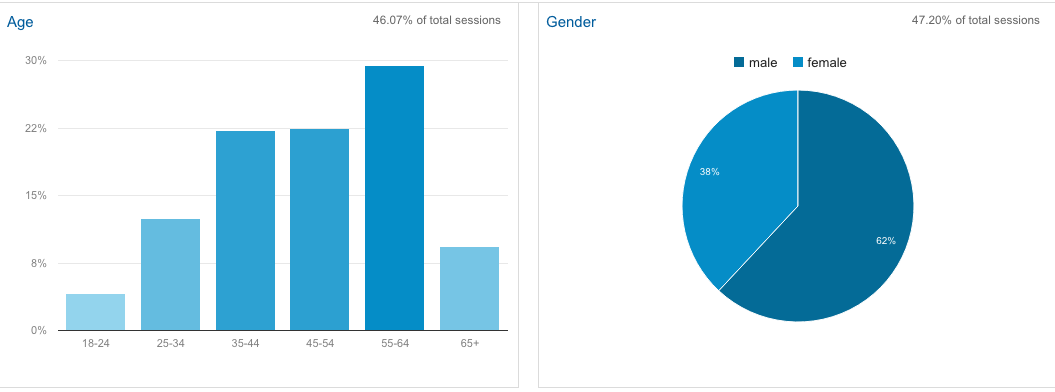
One of the interesting things is that with 1% of the population infected if you consider the impact of spouses and voting age children an organised Hep C "faction" could easily swing 4% of the votes. With an election required by 14th Jan 2017, and therefore likely late next year...
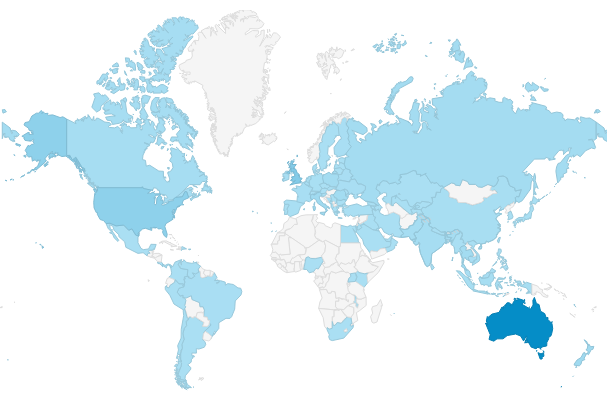
Here is where we all come from - over 100 different countries and counting
ASHM Position Statement On HCV Generics
NSW Health and ASHM (Australian Society of HIV/HBV/HCV Medicine) have joined forces to issue a position statement titled Importation of Generic HCV Drugs. You can follow the link or read the text below:
08 October 2015
Advice for HCV Clinicians
This communique is for clinicians experienced in the management and treatment of Hepatitis C using Direct Acting Antivirals (DAAs), who may be consulted by their patients who are considering importation of generic DAAs. It assumes an understanding of HCV management and new treatments.
Overview of the current status of DAAs in Australia:
All PBS subsidised treatment for hepatitis C in Australia currently consist of pegylated interferon and ribavirin with the addition of a DAA for genotype 1. These combinations are now not widely used due to side effects, duration of treatment and relatively poor cure rates.
Newer DAA regimens that are interferon-free, well tolerated and have shorter duration of treatment are available in the USA and Europe. They are detailed in the current EASL and AASLD evidence-based guidelines.
A number of DAAs have been approved in Australia by the TGA. In 2015, several interferon-free regimens for the treatment of hepatitis C were recommended by the PBAC for inclusion on the PBS, however they are not yet listed. These include:
- Sofosbuvir + Ledipasvir for genotype 1
- Sofosbuvir + Daclatasvir for genotypes 1 and 3
- Sofosbuvir + Ribavirin for genotype 2
- Sofosbuvir + Pegylated interferon/ Ribavirin for genotype 1
- Paritaprevir/r + Ombitasvir + Dasabavir (+/- Ribavirin) for genotype 1
Consumers in Australia can currently access DAAs through a number of mechanisms:
- purchase the drug without subsidy
- participate in a clinical trial
- apply under a compassionate access program (limited places)
- import drugs into Australia for private use under the Personal Importation Scheme
ASHM recommends that any individual considering DAA therapy discuss their options with a clinician experienced in HCV management and treatment with the new DAAs.
Patients may approach clinicians to write them a script for DAAs so that they can purchase them overseas or via the internet due to their current high cost in Australia without subsidy. In these circumstances, it is important they understand that it is the patient that is the personal importer. The Doctor should document fully the patient’s request and understanding and that the doctor is supporting the patient to personally import the medicines. More information on this process is discussed below. Doctors should not advertise or promote these methods of acquisition to patients.
Evidence for the effectiveness of DAAs:
The efficacy of DAAs in the treatment of hepatitis C has been widely established in the international literature and is detailed in the EASL and AASLD evidence-based guidelines.
Discussing importation of generic DAAs with patients:
Patients are allowed to import up to 3 months of medication for their own use under the Commonwealth Government’s Personal Importation Scheme. It is important that patients know that the safety and quality of medicines purchased overseas or over the internet cannot be guaranteed.
It is a legal requirement of this scheme that a patient has a prescription from an Australian registered medical practitioner.
Refer patients to the Hepatitis Australia factsheet Importing Medicines into Australia (May 2015).
Establishing HCV status:
When a patient requests a script for a generic DAA, it is essential to establish their current HCV status. This should be done using a conventional blood sample and laboratory testing.
Current HCV infection is confirmed when the test results are HCV antibody positive and HCV RNA is positive.
Patient HCV assessment:
Once the presence of chronic HCV (HCV RNA +ve) has been confirmed, it is very important in patient assessment to:
- Establish HCV genotype (as this determines the treatment regimen)
- Determine the degree of liver fibrosis (as this influences treatment choice and duration)
- Determine the presence of comorbidities that may affect treatment (e.g. renal disease)
Patients with cirrhosis need ongoing specialist monitoring for complications, including HCC and oesophageal varices. They also need different treatment combinations and more prolonged treatment.
Drug interactions:
The potential for drug interactions with other medication must be considered when DAAs are prescribed. For up to date drug-drug interaction information, go to: http://www.hep-druginteractions.org/ or download the App: HEP iChart.
Compliance with dosing regimen:
It is essential that patients take the right combination of medications for their HCV genotype and stage of fibrosis. They must also take the medication consistently for a sufficient duration in order to optimise the likelihood of viral clearance (SVR or sustained virological response).
Safe injecting strategies:
Patients should be counselled to use safe injecting practices. This will prevent the acquisition of other blood-borne infections and reinfection with hepatitis C.
Monitoring and side effects:
DAAs are generally very safe. Patients should be monitored according to evidence-based guidelines. The key issues are in initial patient assessment (as discussed above).
Patients will also need post treatment assessment including 2 HCV RNAs (PCR) to determine viral clearance. Note that patients with cirrhosis will need lifelong monitoring.
Accessing DAAs through the Personal Importation Scheme
Patients have a number of options to access DAAs, including their purchase in Australia without subsidy. Patients can also personally import the drugs in person or via mail. Details of how to do this can be obtained on the TGA website. A patient can import up to 3 months supply at the one time into Australia using the Personal Importation Scheme.
Generic drugs can be purchased via a number of websites. These drugs have not been evaluated by the TGA. Generic drugs use alternative trade names and packaging and may be a different colour or shape so you must be sure that the drug being purchased is the correct formulation.
Self-importation has some risks associated with it. You do not have the quality protection provided by drugs evaluated and listed on the ARTG. You need to be careful about the veracity of the website. Supply time may vary and the postage time could be delayed if customs investigates the package. Self-importation is legal, but not routine. Having a valid Australian prescription completed by a registered medical practitioner is essential, and completing all the paper work will facilitate the process but patients should allow up to eight weeks for delivery.
How to order Generic DAAs for HCV online:
ASHM does not endorse any specific websites or products. It is up to the individual personally importing the drugs to locate an appropriate website and test the veracity of the supplier. The following are shown as examples only.
FixHepC is a buyers club http://fixhepc.com/getting-treated/how-to-do-it/buyers-club.html that states it will assist with buying, testing and delivery of hepatitis C drugs.
AIDS Drugs Online http://www.aids-drugs-online.com/ has been used to source low-cost generic versions of HIV medications from various overseas manufacturers and they also stock hepatitis C medications. Please be aware that the brand names used on AIDS Drugs Online are not the same as the brands in Australia. For example:
a. Australian brand name: Sofosbuvir
b. AIDS Drugs Online (ADO) brand name: SOFOSBUVIR / Hepcinat
Online suppliers may be only able to supply one of the HCV drugs needed in a regimen, so patients may need to source medicines from more than one source, e.g. the internet and locally through compassionate patient access schemes.
Helpful links:
TGA Personal Importation Scheme https://www.tga.gov.au/personal-importation-scheme
Fix Hep C – website supplying further information on personal importation http://fixhepc.com/
EASL Recommendations on Treatment of Hepatitis C 2015 http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/recommendations-on-treatment-of-hepatitis-c-2015
AASLD HCV Guidance: Recommendations for Testing, Managing and Treating Hepatitis C http://www.hcvguidelines.org/full-report-view
Hepatitis Australia http://www.hepatitisaustralia.com/
For further information:
Contact the ASHM office during office hours on 02 8204 0700.