Displaying items by tag: DAA
AASLD 2017: Generics shown to be bioequivalent to originator brands
Just out of embargo for AASLD 2017 is the rather innocuous sounding Abstract 1078 which says, in brief, that these generic DAAs are inarguably proven the same as the originator DAAs.
Bioequivalent pharmacokinetics for generic and originator Hepatitis C Direct Acting Antivirals
Andrew M. Hill1, Loai Tahat2, Mohammed Khalil Mohammed3, Sanjay Nath4, Rabab Fayez Tayyem3, James A. Freeman5, Ismahane Benbitour7, Sherine Helmy6; 1Department of Translational Medicine, University of Liverpool, Liverpool, United Kingdom; 2Pharmaceutical Research Unit, Amman, Jordan; 3ACDIMA BioCentre, Amman, Jordan; 4Faculty of Medicine, Imperial College London, London, United Kingdom; 5GP2U Telehealth, Hobart, TAS, Australia; 6R&D Project Innovations, Pharco, Cairo, Egypt; 7BEKER Laboratories, Algiers, Algeria
Background: Mass production of low-cost generic directacting antivirals (DAAs) will be required to achieve targets of eliminating hepatitis C (HCV) by 2030. Gilead and Bristol-Myers Squibb have granted voluntary licenses to generic companies to mass-produce the DAAs sofosbuvir and daclatasvir at low cost. However generic manufacturers need to demonstrate bioequivalent pharmacokinetics for their generic DAAs compared to the originator versions, to fulfil World Health Organization standards for pre-qualification.
Methods: Randomised, single-dose, two-way, two-period pharmacokinetic studies were performed in 35-54 healthy volunteers, to compare generic forms of sofosbuvir and daclatasvir with the originator versions. Generics evaluated were from Pharco (Egypt), Beker (Algeria) and Hetero (India), versus originator sofosbuvir (Gilead) and daclatasvir (Bristol-Myers Squibb). All studies were conducted under Good Clinical Practice (GCP). Plasma concentrations of each DAA were assessed over 24 hours. Maximum concentration (Cmax) and Area Under the Curve (AUC) were calculated for each subject. Geometric mean ratios and associated 90% confidence intervals were used to compare each generic DAA with the originator version. Pre-specified limits for the 90% confidence intervals were 80% to 125% of the originator pharmacokinetic concentrations for AUC, and 69-145% for Cmax.
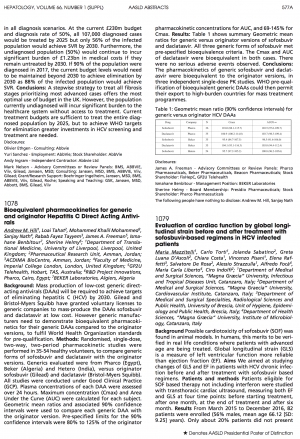
Results: Table 1 shows summary Geometric mean ratios for generic versus originator versions of sofosbuvir and daclatasvir. NB Results from Natco and Virchow came in after the deadline for AASLD submission, but are included here.
Table 1: Geometric mean ratio (90% confidence intervals) for generic versus originator HCV DAAs
| Drug | Company | N | Cmax | AUC0-∞ |
| Sofosbuvir | Pharco | 36 | 101.0 (88.1-115.7) | 103.5 (97.6-109.7) |
| Daclatasvir | Pharco | 36 | 106.9 (100.2- 114.0) |
103.7 (98.3-109.4) |
| Sofosbuvir | Beker | 35 | 95.4 (84.7-107.5) | 98.5 (91.6-106.0) |
| Daclatasvir | Beker | 35 | 35 104.1 (93.1-116.3) | 103.0 (94.4-112.4) |
| Sofosbuvir | Hetero | 54 | 95.7 (97.2- 105.2) | 100.8 (96.2- 105.6) |
| Sofosbuvir | Natco | N/A | 96.1 (81.0-114.0) | 100.7 (94.2-107.8) |
| Daclatasvir | Natco | N/A | 94.5 (83.1-107.4) | 96.5 (87.1-106.8) |
| Sofosbuvir | Virchow | 24 | 94.8 (83.3-107.9) | 95.8 (86.9-105.7) |
All three (now expanded to 5) generic forms of sofosbuvir met pre-specified bioequivalence criteria. The Cmax and AUC of daclatasvir were bioequivalent in both cases. There were no serious adverse events observed.
Conclusions: The pharmacokinetics of generic sofosbuvir and daclatasvir were bioequivalent to the originator versions, in three independent single-dose PK studies. WHO pre-qualification of bioequivalent generic DAAs could then permit their export to high-burden countries for mass treatment programmes.
Disclosures: James A. Freeman - Advisory Committees or Review Panels: Pharco Pharmaceuticals, Beker Pharmaceuticals, Beacon Pharmaceuticals; Stock Shareholder: FixHepC, GP2U Telehealth
Ismahane Benbitour - Management Position: BEKER Laboratories
Sherine Helmy - Board Membership: Presidio Pharmaceuticals; Stock Shareholder: Pharco Pharmaceuticals
The following people have nothing to disclose: Andrew M. Hill, Sanjay Nath
AbbVie's New Hepatitis C Treatment Won't Cure Patient Access Issue
By Priti Krishtel
Last week, the FDA approved AbbVie’s Mavyret—a new hepatitis C virus (HCV) drug that treats all genotypes of the disease and cures more than 90% of patients within just 8 weeks of treatment. This has been reported as a threat to Gilead Sciences’ dominant position in the market, sparking rumors of a potential price war that could lower prices on the infamously expensive treatments.
There is reason to be tentatively optimistic. The initial published price on Mavryet was lower than expected at $13,200 per month—or $26,400 for a full course of treatment—appearing on the surface to be a significant discount from the $94,500 per-treatment course costs of Harvoni, Gilead’s market-leading treatment.
And while the published price is a step in the right direction to getting more people the treatment they need, it isn’t the whole story. The set Mavryet price is reflective of the current market conditions. With behind-the-scenes payer negotiations, the announced price on Mavyret is only an approximately 15% discount to the current net price paid for Gilead’s products.
While AbbVie’s pricing is now the lowest for a curative HCV drug, it is not a radical undercutting of Gilead prices. Millions of Americans with HCV—and tens of millions globally—are already blocked from getting treatment at the current exorbitant prices set by the pharmaceutical industry and the subsequent rationing of treatment approval by payers.
The United States is currently facing an HCV epidemic. There are an estimated 3.6 million Americans with HCV—and that number is expected to rise in the coming years. There is a cure, but every day 48 people in the United States die from HCV, the deadliest infectious disease in America. That’s because 2 out of every 3 Americans who have been diagnosed with HCV do not receive treatment, largely due to the high costs. States such as Louisiana and Pennsylvania are forced to ration treatment to the sickest of patients, and many insurers refuse to cover it because of the high price.
A week out from the FDA’s approval, it is not clear what the introduction of Mavyret will do to change this situation. What is clear, however, is that people should not die of an entirely treatable illness. One of the root causes of the unaffordability crisis is the fact that pharmaceutical companies such as Gilead over-patent drugs, including extending its market monopoly on Sovaldi for at least the next 2 decades.
Gilead’s initial price on Sovaldi has skewed what the market considered a fair price for HCV treatment. I doubt we would see this level of praise for AbbVie now if Gilead hadn’t launched this lifesaving medicine at $84,000 for a single course of treatment.
Until we address the core of the drug pricing problem—unjustified patents—pharmaceutical companies will be able to abuse the system to obtain monopolies on the market and charge astronomical prices that prevent people from getting access to disease-curing medicines.
About the Author
Priti Krishtel is co-founder and co-director of the Initiative for Medicines, Access & Knowledge (I-MAK), a US-based nonprofit group of scientists and lawyers working globally to get people lifesaving medicine. Prior to founding I-MAK, Krishtel obtained her law degree from New York University School of Law worked as a health attorney in the United States, Switzerland and India.
Breaking News: Study Shows HCV DAA Treatment Demonstrates 57% Survival Benefit
"To our knowledge, this is the first large-scale study to demonstrate the effect of newer DAA regimens upon survival. Treatment with 2 commonly used DAA regimens, PrOD and LDV/SOF, was associated with significant improvements in survival within the first 18 months of treatment, compared with demographically and clinically similar untreated HCV-infected controls. Treatment with either PrOD or LDV/SOF was associated with a 57% reduction in mortality, and attainment of SVR was associated with a 43% reduction in mortality….Benefits of treatment at a population level are expected to be substantial…..similar benefit can be expected with other DAA-based regimens"
Effect of Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir and Ledipasvir/Sofosbuvir Regimens on Survival Compared With Untreated Hepatitis C Virus–Infected Persons
Adeel Ajwad Butt Peng Yan Tracey G. Simon Abdul-Badi Abou-Samra
Clinical Infectious Diseases, cix364, https://doi.org/10.1093/cid/cix364
Published: 20 July 2017
Abstract
Background
Interferon-based regimens are associated with a substantial survival benefit for persons infected with hepatitis C virus (HCV). Survival data with direct-acting antiviral agents are not available. We conducted this study to quantify the effect of paritaprevir/ritonavir, ombitasvir, dasabuvir (PrOD) and ledipasvir/sofosbuvir (LDV/SOF) regimens upon mortality.
Methods
In the Electronically Retrieved Cohort of HCV Infected Veterans (ERCHIVES), a well-established national cohort of HCV-infected Veterans, we identified HCV-infected persons initiated on PrOD or LDV/SOF, excluding those with human immunodeficiency virus, hepatitis B surface antigen positivity, hepatocellular carcinoma, or missing HCV RNA or FIB-4 scores. For each case, we identified a propensity score–matched control never initiated on treatment. Primary outcome was survival. Outcomes were assessed using frequency of events, Kaplan-Meier curves, and Cox proportional hazards regression analyses.
Results
We identified 1473 persons on PrOD, 5497 on LDV/SOF, and 6970 propensity score–matched untreated persons. Treated persons were more likely to be obese and have cirrhosis, but less likely to have stage 3–5 chronic kidney disease (CKD), alcohol or drug abuse or dependence diagnosis, and anemia. The proportion of persons who died was higher in the untreated group compared with either treatment group (PrOD, 0.3%; LDV/SOF, 1.4%; untreated controls, 2.5%; P < .001). A significantly larger percentage of treated patients survived to 18 months of follow-up, compared with untreated controls (P < .001). In multivariable Cox regression analysis, treatment with either regimen (hazard ratio [HR], 0.43; 95% confidence interval [CI], .33–.57) and attainment of sustained virologic response (SVR) were associated with significantly lower mortality (HR, 0.57; 95% CI, .33–.99).
Conclusions
Treatment with PrOD or LDV/SOF and SVR are associated with a significant mortality benefit, apparent within the first 18 months of treatment.
ASHM Gearing Up For PBS Listing
ASHM is running the Treatment of hepatitis C with Direct Acting Antivirals course at the Rydges Melbourne, Victoria on Saturday, 20 February 2016. This course is being delivered as part of the Control and Elimination within AuStralia of HEpatitis C from people living with HIV (CEASE) project in partnership with the Kirby Institute.
This full day, face-to-face course is designed to up-skill the primary care-based workforce in the current assessment and treatment of HCV infection, to enable rapid scale-up of interferon-free therapy with direct acting antivirals (DAA) for HCV. The course is designed for HIV prescribers, but may also appeal to General Practitioners, Sexual Health Physicians, OST prescribers, clinicians working in AOD settings, custodial settings and Aboriginal Medical Services.
This is a FREE course, which has been approved for 40 Category 1 RACGP points. A small number of travel scholarships for rural and regional medical practitioners to attend may be available upon request.
I have attached a flyer with further details – Feel free to forward to your colleagues and extended networks. To register for this course please go to the courses page on the ASHM website http://www.ashm.org.au/courses.
Please do not hesitate to contact me via return e-mail if you have any queries.
Kind regards,
Annabelle Kennett
Project Officer
National Policy and Education
ashm | Supporting the HIV, Viral Hepatitis and Sexual Health Workforce
Tel: +61 2 8204 0742 | Fax: +61 2 8204 0782 | E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
LMB 5057 DARLINGHURST NSW 1300 | www.ashm.org.au -