Displaying items by tag: gilead sciences
India grants patent for Sovaldi - Is the Indian Hepatitis C tourism over?
End of Hepatitis C Tourism?
For more than a year, Hepatitis C patients who could not afford the high price tag of the novel Hepatitis C drugs in their respective countries, could go to India and buy the medicines they needed to get well for about $2,000. This window that cured thousands and thousands of patients is about to close, as the result of Indian patent office ruling on drug Sovaldi (400mg sofosbuvir).Gilead Sciences, the pharmaceutical company that makes Sovaldi, failed to show the inventiveness and novelty sufficient to grant a patent on Sovaldi. Since they did not wish to lose such a big market as India, they gave licences to 11 generic manufacturers in India to produce and distribute the low-cost sofosbuvir pills as their plan B (getting a patent being a plan A).
According to Reuters, Gilead appealed against the ruling that refrained Indian patent office from giving them a patent on sofosbuvir molecule. In a dramatic change of heart, Indian patent office just gave Gilead what they wanted all along - a patent. With the plan A in play now, Gilead might choose and forgo plan B - this would mean that there would be no more low-cost sofosbuvir pills to import from India and would leave millions to choose between fetching out more than 80,000$ for the original medicine, or face the lethal consequences of untreated Hepatitis C.
Fix Hep C Buyers Club supply of Hep C drugs is not affected
The Fix Hep C Buyers Club remains one of the strongest providers of low-cost Hepatitis C medicines. Since sofosbuvir the buyers club provider is supplied from China, not India, the supply chain will not be affected and every Hepatitis C patient can seek help they need by contacting us (click here).Why did the Indian patent office change its mind?
In direct contradiction to its earlier order, the Controller General of Patents, Designs and Trademark granted American pharmaceutical company Gilead Sciences the patent for the blockbuster Hepatitis C drug Sofosbuvir (brand name Sovaldi) in India. An application for the same patent was first rejected in January 2015 as lacking inventiveness and novelty.On Monday, however, the patent office dismissed all pre-grant oppositions and stated that it found, “claimed compounds are novel, inventive and patentable under Patents Act.” The decision is a major blow to the access to drug movement, said Leena Menghaney, South Asia head of Médecins Sans Frontières (MSF). “There has been excessive pressure building up on the Indian government to dilute the independent functioning of the patent office to ensure that patent claims are granted far more easily to U.S. firms. In the process, the patent office has completely ignored recent proceedings in the U.S. against Gilead regarding the same application which have been found to infringe two of Merck’s patents, clearly defeating Gilead’s claim that its application on the drug was novel,” added Ms Menghaney.
How obtaining a patent works
“These are very scary times for the patient communities globally who rely on affordable generic medicines coming from India. The government’s “Make in India” campaign seems to be only for foreign companies and not for Indian generic industry which has been the lifeline for people across the world,” said Loon Gangte with the Delhi Network of Positive People
Gilead, in a statement, welcomed the move, but said it will have no impact on availability of the compound, which is already licensed to 11 generic manufacturers in India for distribution in 101 developing countries.
Sofosbuvir patent issue goes beyond patent office
Another key application on sofosbuvir is pending before the Kolkata patent office and several oppositions to its grant have been filed by patient and public interest groups. Stating that the case had been decided outside the merit of the technical and legal issues, Tahir Amin Co-Founder and Director of Intellectual Property Initiative for Medicines, Access & Knowledge (I-MAK) said that the organisation would appeal against the decision. “The Indian patent office has had to deal with a lot of external influences around this case, especially since the initial decision last year. Clearly the decision has been taken outside the realm of the patent office. The decision has not been reasoned properly and there are a lot of discrepancies. The interpretation of the law as it is intended has not been applied and we will be appealing against it.”Get your Hepatitis C medicines today at Fix Hep C Buyers Club
Pharmaceutical companies are trying ever harder to restrain the production of low-cost Hepatitis C medicines on all fronts. For them, a Hepatitis C patient is worth $80,000 or more. For us at Fix Hep C Buyers Club, Hepatitis C patient is worth saving because we consider it morally a right thing to do; this is why we have obtained a legal and safe way for Hepatitis C patients to obtain the medicines they need for less than $2,000. Please do contact us with the nature of your disease and Dr. Freeman will advise you on the best way of treatment, and what is even more important, we will deliver the treatment to your doorstep as soon as possible.Hepatitis C is no longer a death sentence. Why are people still dying because of it?

New age Hepatitis C medicines are more than just a medical wonder. They gave humanity an ability to save lives.
How good are we at saving those lives?
More people die of Hepatitis C than HIV/AIDS
Hepatitis C is a serious disease that ultimately results in death of patients. Approximately 500,000 people die from Hepatitis C and related illnesses in 2013 alone, more than 20,000 of them were US citizens. To put it in perspective: HIV/AIDS has longed been talked about as a very serious disease with a disastrous death toll. However, according to Dr. Laura J. Martin of WebMD nowadays more people die from Hepatitis C than from HIV/AIDS.
Today, there are more than 3.2 million of Hepatitis C patients in the US alone.
In late 2013, however, humanity had a break-through that should by all accounts drastically change lives of people living with Hepatitis C. A new drug, Sovaldi (400mg sofosobuvir), was approved in December 2013 on the US market.
With it, more than 90% of people with Hepatitis C can be cured. But are they really being cured?
Standard Interferon-based treatment
For any disease to be deemed a very problematic one, there are two conditions:
- Disease is serious (causes severe injuries or death)
- There is a lack of efficient cure
Polio, for example, was a very serious disease with a disastrous outcome. However, after discovering an efficient polio vaccine, the number of patient and number of death relating to polio was reduced dramatically.
Hepatitis C prior to 2013 was a very problematic disease because it caused death via liver cirrhosis and liver cancer, and the only treatment we had was 50% efficient.
Hepatitis C patients were put on 6-months long interferon-based treatment which consisted of injecting oneself with interferon and taking additional oral medicines such as ribavirin (antiviral molecule). Nonetheless, for 1 out of every 2 patients treated the treatment has been proven to be unsuccessful.
There was a need for an efficient cure. Newly-discovered sofosbuvir molecule was the answer.
New age Hepatitis C treatment - Sofosbuvir-based medications
With the launch of Sovaldi and Harvoni medicines by a company Gilead Sciences, humanity finally attained a very effective cure for Hepatitis C. Being an all-oral regimen, sofosbuvir pills are taken on a daily basis for 12-weeks (standard treatment), have mild side effects and, above all, more than 95% cure rate. This is what in pharmaceutical industry refer to as a game-changer. Now almost everyone can be cured and Hepatitis C suddenly became an easily curable disease.
Does anybody die of Hepatitis C now?
Simple answer is 'YES'. While the number of deaths has decreased from 500,000 per year, there are still hundreds of thousands of people dying every year. The reason: Hepatitis C.
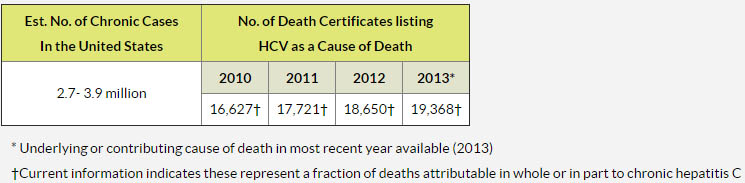
But if we know Hepatitis C is so easily treatable nowadays, why are people still dying?
Pharmaceutical industry is a profitable business (Money>Patients)
When we spoke about Harvoni and Sovaldi being a game-changer in industrial industry, it was meant more in profits than in saving lives. Here are two simple reasons why people even in the developed world are still dying of Hepatitis C.
- Original Sovaldi (400mg sofosbuvir) costs $80,000 per treatment (US prices)
- Original Harvoni (90mg ledipasvir/400mg sofosbuvir) costs $94,500 per treatment (US prices)
With this in mind, let us calculate the US Hepatitis C market. If we know there are 3,2 million Hepatitis C patients, each in need of an $80,000 cure, the total comes to staggering number: Gilead Sciences is looking to sell more than $250 billion worth of Hepatitis C medicine to patients who can die without it.
Here is a horrifying realization. We have people who will die without the cure. We have the cure. But people who are dying cannot afford the cure because it is priced extremely high. 'What have we come to as a society?' is the right question here.
Way to get Hepatitis C medicines without having to pay massive sums of money
Gilead Sciences, company that markets Sovaldi and Harvoni, offered licences to Indian manufacturers to produce generic version of sofosbuvir-based medicines. In short, India refused to recognise a level of innovation for sofosbuvir molecule that would grant Gilead Sciences a patent and monopoly over Hepatitis C market in India.
This created a loophole. This loophole is now saving lives.
FixHepC Buyers Club to the rescue
All around the world there are Hepatitis C patients that will die without getting the cure - and they are not getting it because the prices of the drugs are so extremely high. This is where FixHepC Buyers Club comes in.
It is our mission to deliver life-saving Hepatitis C medicines to your doorstep for a negligible cost. We have set up a supply chain consisting of sofosbuvir production, packaging and distribution across the world. It is our hope this will bring down the Hepatitis C death toll under 100,000 and that in near future Hepatitis C death cases will be as few in number as possible with sofosbuvir-based medication.
We strive to deliver generic Harvoni anywhere on the planet for less than $2,000 per treatment in about 2-3 weeks. With this prices, we could cure all Hepatitis C patients in the US for less than $7 billion.
Are thing in Hepatitis C market likely to change?
Hardly. Pharmaceutical industry holds on to patents for drugs that last for 20- to 25-years. During this time, the prices of original Sovaldi and Harvoni will be extremely high, and Hepatitis C patients don't have 20 or more year to wait for patents to expire.
If you have Hepatitis C, act now. The FixHepC Buyers Club will help you every step of the way, from getting the necessary medicines to monitoring your treatment success. You can call us or send us an email with your inquiries to This email address is being protected from spambots. You need JavaScript enabled to view it..
