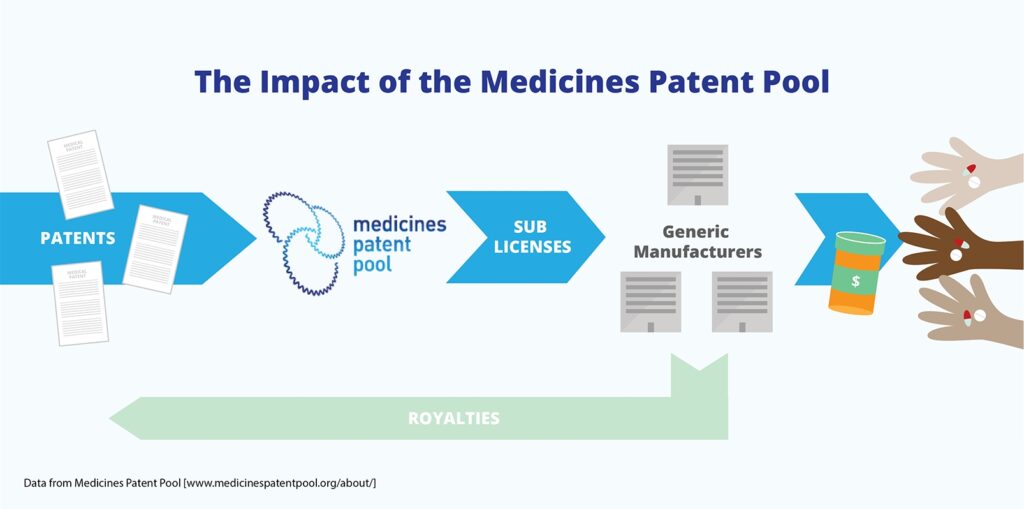
There is some exciting news from the Medicines Patent Pool in that Mylan have signed on to produce a generic glecaprevir/pibrentasvir. https://medicinespatentpool.org/mpp-media-post/the-medicines-patent-pool-and-mylan-sign-agreement-to-scale-up-access-to-first-generic-version-of-hepatitis-c-treatment-glecaprevir-pibrentasvir/ This is really significant for areas of Africa where the subtypes of HCV are very resistant to DAAs, for patient with renal failure and for patients who have failed one or more DAA […]
Tag Archives: maviret
News Flash MAVIRET® (glecaprevir/pibrentasvir) was listed on the PBS in Australia today. AbbVie is pleased to announce that MAVIRET is to be listed on the PBS on 1 August 2018 for the treatment of chronic hepatitis C virus (HCV) infection in adults. MAVIRET is a new 8 week pangenotypic treatment for treatment-naïve non-cirrhotic HCV adult […]
By Priti Krishtel Last week, the FDA approved AbbVie’s Mavyret—a new hepatitis C virus (HCV) drug that treats all genotypes of the disease and cures more than 90% of patients within just 8 weeks of treatment. This has been reported as a threat to Gilead Sciences’ dominant position in the market, sparking rumors of a […]