Just out of embargo for AASLD 2017 is the rather innocuous sounding Abstract 1078 which says, in brief, that these generic DAAs are inarguably proven the same as the originator DAAs.
Bioequivalent pharmacokinetics for generic and originator Hepatitis C Direct Acting Antivirals
Andrew M. Hill1, Loai Tahat2, Mohammed Khalil Mohammed3, Sanjay Nath4, Rabab Fayez Tayyem3, James A. Freeman5, Ismahane Benbitour7, Sherine Helmy6; 1Department of Translational Medicine, University of Liverpool, Liverpool, United Kingdom; 2Pharmaceutical Research Unit, Amman, Jordan; 3ACDIMA BioCentre, Amman, Jordan; 4Faculty of Medicine, Imperial College London, London, United Kingdom; 5GP2U Telehealth, Hobart, TAS, Australia; 6R&D Project Innovations, Pharco, Cairo, Egypt; 7BEKER Laboratories, Algiers, Algeria
Background: Mass production of low-cost generic directacting antivirals (DAAs) will be required to achieve targets of eliminating hepatitis C (HCV) by 2030. Gilead and Bristol-Myers Squibb have granted voluntary licenses to generic companies to mass-produce the DAAs sofosbuvir and daclatasvir at low cost. However generic manufacturers need to demonstrate bioequivalent pharmacokinetics for their generic DAAs compared to the originator versions, to fulfil World Health Organization standards for pre-qualification.
Methods: Randomised, single-dose, two-way, two-period pharmacokinetic studies were performed in 35-54 healthy volunteers, to compare generic forms of sofosbuvir and daclatasvir with the originator versions. Generics evaluated were from Pharco (Egypt), Beker (Algeria) and Hetero (India), versus originator sofosbuvir (Gilead) and daclatasvir (Bristol-Myers Squibb). All studies were conducted under Good Clinical Practice (GCP). Plasma concentrations of each DAA were assessed over 24 hours. Maximum concentration (Cmax) and Area Under the Curve (AUC) were calculated for each subject. Geometric mean ratios and associated 90% confidence intervals were used to compare each generic DAA with the originator version. Pre-specified limits for the 90% confidence intervals were 80% to 125% of the originator pharmacokinetic concentrations for AUC, and 69-145% for Cmax.
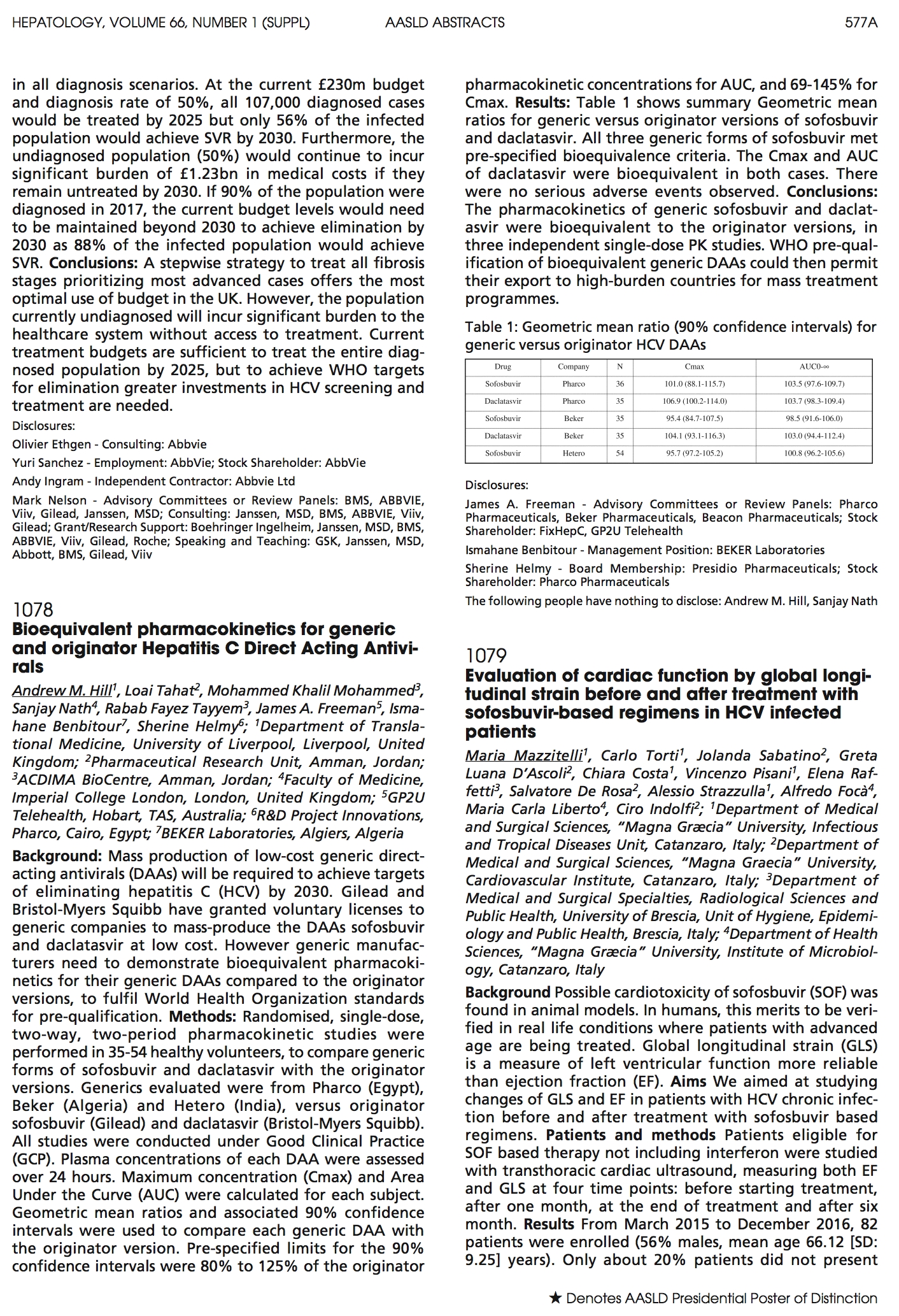
Results: Table 1 shows summary Geometric mean ratios for generic versus originator versions of sofosbuvir and daclatasvir. NB Results from Natco and Virchow came in after the deadline for AASLD submission, but are included here.
Table 1: Geometric mean ratio (90% confidence intervals) for generic versus originator HCV DAAs
| Drug | Company | N | Cmax | AUC0-∞ |
| Sofosbuvir | Pharco | 36 | 101.0 (88.1-115.7) | 103.5 (97.6-109.7) |
| Daclatasvir | Pharco | 36 | 106.9 (100.2- 114.0) |
103.7 (98.3-109.4) |
| Sofosbuvir | Beker | 35 | 95.4 (84.7-107.5) | 98.5 (91.6-106.0) |
| Daclatasvir | Beker | 35 | 35 104.1 (93.1-116.3) | 103.0 (94.4-112.4) |
| Sofosbuvir | Hetero | 54 | 95.7 (97.2- 105.2) | 100.8 (96.2- 105.6) |
| Sofosbuvir | Natco | N/A | 96.1 (81.0-114.0) | 100.7 (94.2-107.8) |
| Daclatasvir | Natco | N/A | 94.5 (83.1-107.4) | 96.5 (87.1-106.8) |
| Sofosbuvir | Virchow | 24 | 94.8 (83.3-107.9) | 95.8 (86.9-105.7) |
All three (now expanded to 5) generic forms of sofosbuvir met pre-specified bioequivalence criteria. The Cmax and AUC of daclatasvir were bioequivalent in both cases. There were no serious adverse events observed.
Conclusions: The pharmacokinetics of generic sofosbuvir and daclatasvir were bioequivalent to the originator versions, in three independent single-dose PK studies. WHO pre-qualification of bioequivalent generic DAAs could then permit their export to high-burden countries for mass treatment programmes.
Disclosures: James A. Freeman – Advisory Committees or Review Panels: Pharco Pharmaceuticals, Beker Pharmaceuticals, Beacon Pharmaceuticals; Stock Shareholder: FixHepC, GP2U Telehealth
Ismahane Benbitour – Management Position: BEKER Laboratories
Sherine Helmy – Board Membership: Presidio Pharmaceuticals; Stock Shareholder: Pharco Pharmaceuticals
The following people have nothing to disclose: Andrew M. Hill, Sanjay Nath

