|
Welcome,
Guest
|
×
Advanced Search
×
Search Results
Searched for: treatment
| 03 Dec 2018 03:10 | |
|---|---|
|
Ron I have also been trying to get the word out here in Southern California about Dr. Freeman and the FixHepC Buyers Club and have been shocked by the lack of interest. I printed up flyers and spoke to the head of a local AIDS organization. I have given them information to distribute. I have offered to help anyone through the process. Perhaps it is because I am a 68 yr old woman. I'm starting to feel invisible. My doctor refuses to give out information or even mention Dr. Freeman. He says it is an insurance liability issue. Well I will keep on trying.
Congratulations on your successful treatment. My heath has continued to improve. I did have a root canal 6 months after EOT. Another person on this site also developed dental issues after finishing treatment. Make sure you get regular dental check ups this next year.
Category: End of Treatment - EOT
|
|
| 02 Dec 2018 20:38 | |
|---|---|
|
Hi mrcleanrt,
Congratulations on your result, and... Hi Ron, Thank you for the kind words and punching a small hole in the stigma of Hep C by putting your name to it. With any luck you will see the same sort of fibrosis regression we've seen in other patients. Hazel, for example, started at 40kPa and is now down below 8kPa. It's a privilege to be able to assist patients to get access to treatment. Thanks for trying to get the word out. I'm sure you've seen the film the Matrix. The deeper you delve into the world of Big Pharma or Big Business the more you realize the world as you think you know it is just a veneer - there are lots of deeper layers...
Category: End of Treatment - EOT
|
|
| 27 Nov 2018 07:28 | |
|---|---|
|
Hello Kansuke, your test results are quite encouraging, your liver fibrosis is low and Genotype 2 is one of the easiest genotypes to treat. Regarding the needed prescription, of course you know that the decision tool is just for general guidance and that the doctor is the one who will ultimately decide which medication is right for you. However, in terms of generic medications, if you take a look at the international treatment guidelines fixhepc.com/genotype-specific-hepc-treaments.html they indicate that treatment naive Genotype 2 patients with low fibrosis (such as yourself) should be treated for 12 weeks without Ribavirin with either one of the following medications (both of them are extremely effective with about 95% cure rates) :
1. Sofosbuvir 400 mg + Daclatasvir 60 mg (Sovaldi® + Daklinza®), which is REDEMPTION-2 on the FixHepC website fixhepc.com/redemption-etrials 2. Sofosbuvir 400 mg / Velpatasvir 100 mg (Epclusa®), which is REDEMPTION-4 on the FixHepC website fixhepc.com/redemption-etrials Best of luck to you.
Category: New to Forum
|
|
| 26 Nov 2018 11:43 | |
|---|---|
|
Hello Songbird,
You can't really compare Maviret to Sofosbuvir based regimens and say what is true for the most potent NS3/4A and NS5A agents ever invented is applies to NS5A/NS5B combinations. HCV represents a heterogeneous quasispecies - the population in any patient exhibits a great deal of variability. It stands to reason some is easy to kill (call them ordinary infantry), some is harder to kill (call them special forces with body armour) and some is harder again to kill (call them tankers). Rapid viral suppression represents two distinct things. First we are looking at the decline of the easy to kill population (and this is relatively irrelevant) but secondly, and perhaps more importantly, it represents the window period where there are viable replicons and a drug-driven selective pressure on resistance. In the context of drugs being present, we are selecting for the resistant forms to replicate, and one more mutation could be enough to overcome the EC50 of the drugs we are using. We do know from HIV that continuing to use drugs where the patient has a viral load leads to the development of resistance. We are lucky that the drugs work as well as they do. To me, the issue with fibrosis is that the lower blood supply of fibrotic tissue leads to lower tissue concentrations and thus the potential for the drugs to be present at < EC50. The same goes for BMI - we have certainly seen Daclatasvir toxicity in < 50kg patients and treatment failure more common in > 150kg patients. This is a problem with the one size dose fits all model currently in use. Gilead's research in children adjusts the dose down based on bodyweight (makes perfect sense) but there is no dose adjustment UP for larger people. There is dose adjustment up for many other drugs - antibiotics, anaesthetics, ...
Category: Q & A
|
|
| 25 Nov 2018 08:45 | |
|---|---|
|
Hello tototo,
The baseline GT1 cure rate for Maviret is very high. The company says 99% which is probably optimistic but it's almost certainly as high or higher than Harvoni which is mid-nineties. So you are GT1a, have previous failure to PEG/Riba (not exactly a failure because you did not do the full course), and are F1/F2, and have HCV RNA <15 but still detected at 4 weeks... And AFAIK there is no clinical trial that I'm aware of that informs a specific answer for a large group of patients with your specific profile. Over here you will find an ongoing discussion that would be relative to you if you were taking Harvoni fixhepc.com/forum/questions-and-answers/...eks-or-24-weeks.html And over here you will find an ongoing discussion about how cure rate improves with duration of treatment fixhepc.com/forum/experts-corner/2062-sh...e-for-treatment.html Questions: how long are you scheduled for, and how good is your insurance? If you're scheduled for 8 weeks your SVR12 probability has probably fallen from high 90s to lower 90s If you have good insurance then retreatment for longer with Maviret+Sof or Vosevii will have a mid 90s probability of success so it's not the end of the world if the first treatment does not work (and the odds are still very much that it WILL)
Category: DAA Side Effects
|
|
| 25 Nov 2018 08:28 | |
|---|---|
|
Hello Songbird,
This study was published in 2015 and the conclusions came from pooled data from small clinical trials. Here's a much larger VA study 4,365 patients all taking Harvoni that followed this and was published April 2016. www.researchgate.net/publication/3016714..._C_Infected_Patients pp408 Significantly lower SVR rates were observed in those receiving LDV/SOF who had a 4-week on-treatment detectable HCV RNA <15 IU/mL compared to those who were undetectable by week 4. pp410 Four-week on-treatment HCV RNA was an independent predictor of SVR when included as a variable in the models (Supporting Table S2). Having a detectable HCV RNA <15 IU/mL was associated with reduced odds of SVR compared to undetectable in the full model (OR 0.40, 95% CI 0.29-0.56, P<0.001)and in the model limited to those who completed 12weeks of treatment (OR 0.38, 95% CI 0.25-0.58, P<0.001). pp413 In this analysis, 4‐week viral kinetics predicted SVR with a greater effect in those who received LDV/SOF. Significant reductions of 10.5% and 7.1% in SVR rates were observed in patients receiving LDV/SOF and LDV/SOF+RBV, respectively, who had a detectable 4‐week HCV RNA ≥15 IU/mL compared to those with undetectable 4‐week HCV RNA. In patients who completed 12‐week courses of LDV/SOV there remained a significant 6.4% reduction in SVR rates between those with detectable 4‐week HCV RNA ≥15 IU/mL and those with undetectable 4‐week HCV. For those who completed 12 weeks of LDV/SOF+RBV the difference in SVR rates was no longer statistically significant. In multivariable analysis, having a HCV RNA ≥15 IU/mL after 4 weeks of treatment was associated with a 60% reduced odds of achieving SVR for the cohort and a 62% reduced odds of achieving SVR for those who completed 12 weeks of treatment. The VA guidance recommends obtaining 4‐week HCV RNA testing, thus providing a large sample size to evaluate this variable which has not generally been assessed in prior LDV/SOF studies. The clinical implications of this finding on treatment decisions, such as potentially adding RBV or extending treatment duration based on 4‐week on‐treatment HCV RNA, warrant further study. Call it an inconvenient truth if you like, but a truth it is. I have 3460 patients at last count and initial slow responders used to feature disproportionately in the retreatment group. They feature less now because I extend or modify their treatment. You may find this interesting in terms of digging into the science of what needs to be done to get to cure: www.bhiva.org/file/lOvBacnRQiHzM/ValeriaCento.pdf You may also note that this is an early study based on small numbers and concludes in a 1/2 and 1/2 way saying
Category: Q & A
|
|
| 24 Nov 2018 11:19 | |
|---|---|
|
Thank you Dr James,
In 2001 I did interferon with ribavirin but could only endure 2 months if I well remember. I am F1/F2 as of beginning of treatment. Do you reckon I have good chance to clear at this rate?
Category: DAA Side Effects
|
|
| 24 Nov 2018 10:59 | |
|---|---|
|
This is a <15 result but still detected. It is a good result at 21 days and your liver engymes are great. Your urea levels depend on how much you drink, yours is low and this just means you drink a bit more water than average.
For most people who are treatment naive and low fibrosis 8 weeks of Maviret is enough. The doctor who prescribed it would be the best person to ask as I don't know your full background and history.
Category: DAA Side Effects
|
|
| 24 Nov 2018 10:43 | |
|---|---|
|
Hi Dr James,
Thank your message. I realized that I was having an alergic reaction to Lexotan, a pill I have used in the past for insomnia. I found out I had a few and used them. Well that is sorted out. Now, I just got the BW test results I did on November 15th,exactly 21 days after starting with Mavyret. I am a bit confused as to (detected or not detected???) but all in all it looks like this is working very, very, very well! This is how it goes HCV RNA QUANTITATIVE REAL TIME PCR Performed using real time Polymeras chain reaction Reportable Range:15 IU/ml to 100,000,000 IU.ml (1.18 Log IU/ml to 8.00 IU/ml VALUE HL REFERENCE RANGE HCV RNA QUANTITATIVE REAL TIME PCR <15 DETECTED A NOT DETECTED HCV RNA QUANTITATIVE REAL TIME PCR <1.18 DETECTED NOT DETECTED AST 17 ALT 8 UREA NITROGEN 6 (out of range) Is it detected or NOT detected? Dr James, do you think I will be in the clear at the end and stick to the 8 weeks protocol or should I reinnforce and extend to 12 weeks or 10 weeks. This is working right. 21 days later? I appreciate your dedication and kindness in being of support here since I started my treatment, I truly do. Gratefully, T
Category: DAA Side Effects
|
|
| 24 Nov 2018 08:41 | |
|---|---|
|
Hello Songbird,
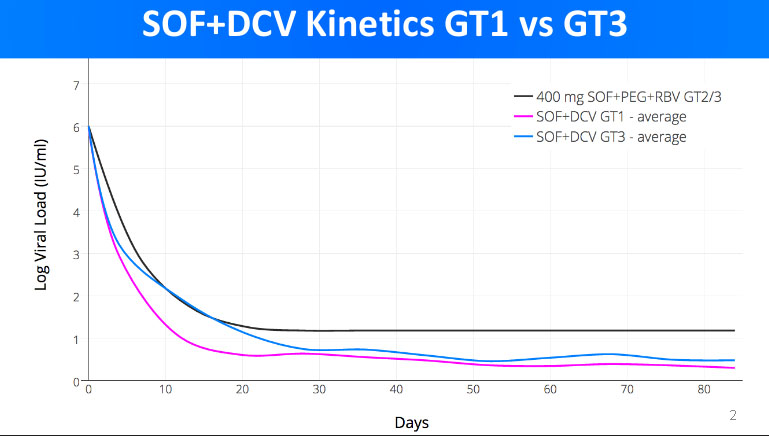
I'm not talking about EOT results. I'm talking about on treatment kinetic decay, or more specifically rate of kinetic decay. If you have a look here fixhepc.com/component/k2/itemlist/tag/In...Congress%202016.html you will see my original presentation to EASL in 2016. It contains 2 graphs about the kinetic decay of the viral load. The first is a comparator to SOF+PEG+RIBA to SOF+LDV and SOF+DCV. The At the time I had some unique data, namely the kinetic decay for SOF+DCV in patients with both GT1 and GT3 as well as SOF+LDV for GT1. The upshot is that SOF+DCV produces a slightly faster kinetic decay than SOF+LDV in GT1 patients, and that, for SOF+DCV the GT3 kinetic decay is considerably slower than GT1. The significance of the rate of kinetic decay is that logically we need to get to ~0-100 total virions left to get cure. This is the infective dose and also way too small to measure as you have 1000 5ml tubes of blood in you so even if the virus was completely blood born, and the cure remainder was <100 viruses only 1 in 10 blood tubes would have a single virus in it. Anyway, it follows that if you are going down slower you need to treat for longer to have the same overtreatment insurance buffer.
Category: Q & A
|
|
| 24 Nov 2018 06:32 | |
|---|---|
|
Doctor when you say:
"We know that about 22% of patient will detected at 4 weeks, and also that these people form 44% (our data) or 50% (US VA data) of the treatment failures Being still detected at 12 weeks is, therefore, a worry. I had read some recent research awhile ago and actually I had seen here that you also had copied it for perusal where the conclusion now states; "Contrary to past experience with interferon-containing treatments, low levels of quantifiable HCV RNA at [end of treatment] do not preclude treatment success," the study authors concluded. fixhepc.com/forum/viral-load-and-svr/785...ot-predict-cure.html Also I had read this not long ago: Conclusion: Presence of DNQ results at EOT does not predict HCV relapse, independently to the sensitivity of the test used. In less than half of DNQ samples, HCV-RNA detection is confirmed by the US test with LLQ of 4UI/ml. Frequency of DNQ samples decreases during and after treatment, suggesting to reflect treatment efficacy and not aspecific RNA carry over in PCR livertree.easl.eu/easl/2017/internationa...table.html?f=m3t4035 Am I possibly missing what the latest data seems to be saying? Thx S
Category: Q & A
|
|
| 23 Nov 2018 10:15 | |
|---|---|
|
Hello Elizabeth,
Without knowing the exact details it's hard to give proper advice but...
PS: If you have to pay for the PCR I would suggest saving that money (it adds nothing to the cure rate) and spending it on more medication to extend the treatment duration. While showing up undetected is nice, it is pretty meaningless in terms of treatment decisions ie treat for longer or not. You mother has responded slowly so has pretty resistant virus.
Category: Q & A
|
|
| 23 Nov 2018 00:58 | |
|---|---|
|
Hello Elizabeth, welcome to the forum. Just because the test gives an untedectable result, doesn't mean that the patient is cured and should stop the medication. The virus can come back after an undetected result if the patient doesn't continue treatment for the duration indicated in the prescription. Also, GT3 is the hardest to treat, your mum should continue her 24 weeks treatment especially that she was detected at 12 weeks. I know that Ribavirin is not the easiest drug to take, but it will be all worth it at the end. If she continues her 24 weeks treatment, there is an excellent chance she will be cured.
Category: Q & A
|
|
| 22 Nov 2018 12:23 | |
|---|---|
|
Hi Tototo,
I just want to chime in here for your benefit. I really hope the Marivet works for you. If it does not, please contact Dr. James here. He will prescribe other medications and help you to be cured. He can help you obtain generic medications that are the equivalent Harvoni/Epclusa or Marivet. He is the leading expert on the treatment of Hep C. I took generic Harvoni and I am close to 3 years now being cured.
Category: Experts Corner
|
|
| 22 Nov 2018 12:19 | |
|---|---|
|
Hello tototo,
It is almost certainly a drug allergy. Some patients with liver disease get itchy due to the build up of toxic stuff. These people generally get better with treatment. With any drug, stomach upsets and rashes are the two commonest side effects. In terms of treatment success it's neither a good or a bad sign, unless it means you can't complete your full treatment. The options are: 1) Stop the medications (and you will probably relapse) 2) Take an antihistamine like Claratyne (loratidine), Zertec (cetirazine) Phenergan (promethazine) which may help settle it down - for other antihistamines check interactions at www.hep-druginteractions.org/checker (but these 3 are all fine with Maviret) 3) Get hold of some generic Epclusa and swap over to taking that to get the treatment duration out to 12 weeks.
Category: DAA Side Effects
|
|
Time to create page: 1.045 seconds