Home › Forums › Main Forum › FixHepC Admin › Indian Generic Velpatasvir Launched (PENDING VERIFICATION)
- This topic has 11 replies, 4 voices, and was last updated 9 years, 3 months ago by
 tweakmax.
tweakmax.
-
AuthorPosts
-
13 October 2016 at 5:21 pm #23799
ADMIN NOTE:
This announcement may be premature. Although there is no doubt this will appear in India in due course, and Hetero have indeed registered the trade name Velasof, it appears that Velasof is yet to be approved by the DCGI, and that there is no commercial availability at this time.
It appears that the image may be pre-production samples sent to Nepal, which historically is similar to previous events.
We will update this thread in due course, but it has been locked for now.
——
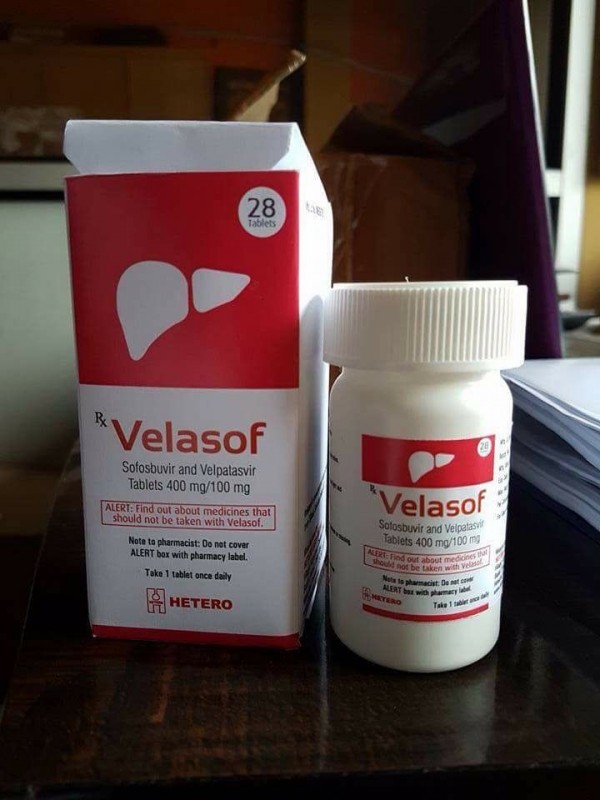
Hetero Launched Generic Sofosbuvir Velpatasvir in several markets today. Its named Velasof.
Velpanat from NATCO will launch shortly.
Approx price is 21000INR in India for 28tab bottle.
Velasof photo attached.
Update to clarify comments below.Please note Velpatasvir is not available for purchase at any retail or distribution center in India yet. Both Hetero and NATCO are gearing up for the earliest time in market.
Attachments:13 October 2016 at 9:40 pm #23803Fantastic news

GT1a Dec14 F2/8.7 VL 900000-2.5M
Jan16 Hepcivir-L MonkMed/Redemption
Baseline: VL 913575 Alt 76 Platelets low
Wk2 VL1157 Alt 23
DET Wk 8 VL 32 Alt19 ‘In the slow lane’
June16 Fibro 5.7 F0/1 LIF 1.5
Wk 11 VL<12 Alt 13 Det/Unq
Extending tx 12 wks Mylan Sofo/Dac MonkMed
Wk 14 VL <12 Det/Unq
Wk 16 VL UNDETECTED
Wk 22 + 4 Wks Sunprevir FixHepC
Wk 24 UNDETECTED Alt 13
Wk 12 post tx SVR12 Wk 26 SVR24
Thank-you Tim, Dr Debasis @ MonkMed & Dr Freeman @ Fix HepC14 October 2016 at 7:35 am #23824MonkMed wrote:Hetero Launched Generic Sofosbuvir Velpatasvir in several markets today. Its named Velasof.
Velpanat from NATCO will launch shortly.
Approx price is 21000INR in India for 28tab bottle.
Velasof photo attached.
So india already approved?
14 October 2016 at 2:25 pm #23840Has anyone here bought and use it yet?
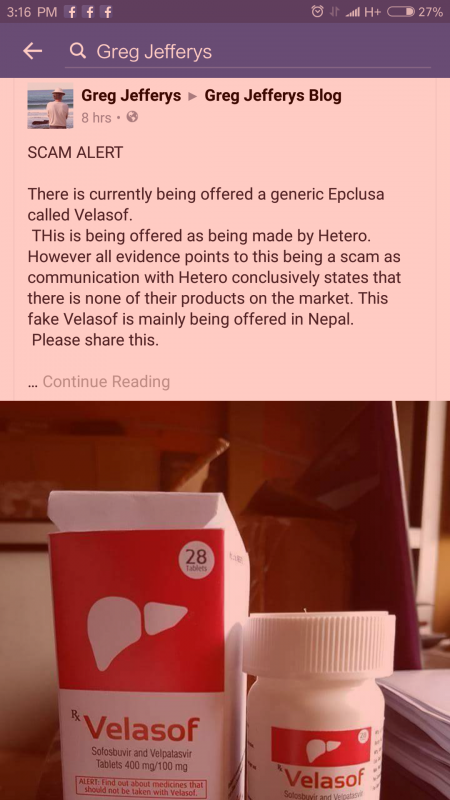
15 October 2016 at 8:18 am #23875According to Greg Jeffery’s fb, India haven’t release velpatasvir yet. Can anyone ascertain whether it has been released?
15 October 2016 at 11:00 am #23882I believe Monkmed did that at the top of this thread Tweakmax. I can also see Bull Pharmachem has Velasof listed on Indiamart.
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 15 October 2016 at 11:17 am #23885Gaj wrote:
15 October 2016 at 11:17 am #23885Gaj wrote:I believe Monkmed did that at the top of this thread Tweakmax. I can also see Bull Pharmachem has Velasof listed on Indiamart.
This is what Greg said, in the attachment
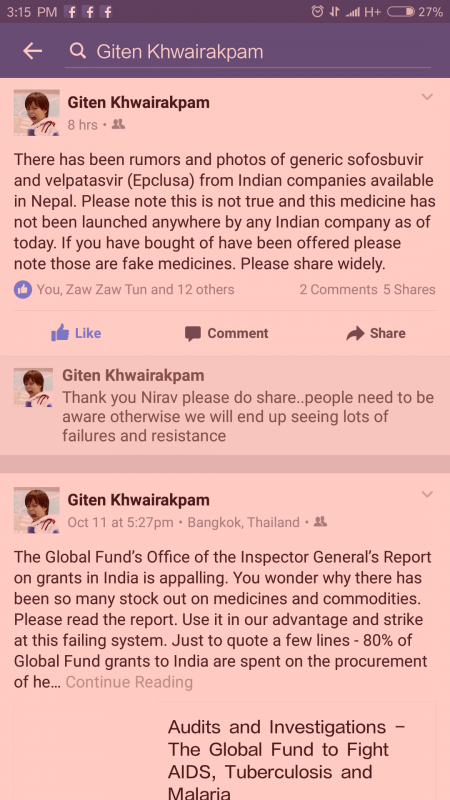
Attachments:15 October 2016 at 11:18 am #23887This is from giten. His info about the generics in India is reliable.
Attachments:15 October 2016 at 11:24 am #23888Thankyou tweakmax,
You apparently have more information than I do.
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 15 October 2016 at 11:32 am #23889Gaj wrote:
15 October 2016 at 11:32 am #23889Gaj wrote:Thankyou tweakmax,
You apparently have more information than I do.
Not really. I am curious to know about the status of generic velpatasvir in India. Tats why I ask whether it has been approved or not.
I remembered harvoni took quite a while to come out and I always checked the minutes of the Indian authorities to see whether it has been approved or not
15 October 2016 at 11:32 am #23890I think it is this one: http://cdsco.nic.in/forms/list.aspx?lid=1880&Id=32
15 October 2016 at 11:50 am #23891http://cdsco.nic.in/forms/list.aspx?lid=2034&Id=11
No mention of velp on the approved drug list yet
-
AuthorPosts
- You must be logged in to reply to this topic.