Friday, 07 July 2017 20:07
The Lancet - What is the impact of treatment for hepatitis C virus infection?
Written by Super User
This article was just published in the Lancet The introduction of direct-acting antiviral (DAA) medicines in 2013 revolutionised the treatment of chronic hepatitis C virus (HCV) infection. The efficacy of DAA therapy is impressive—in many clinical trials HCV cannot be detected by…
About 1 in 5 patients taking Direct Acting Antivirals will get insomnia. Here is a post of mine from some years ago about how to manage it. Insomnia is a common and often frustrating problem. Part of the reason it's frustrating is…
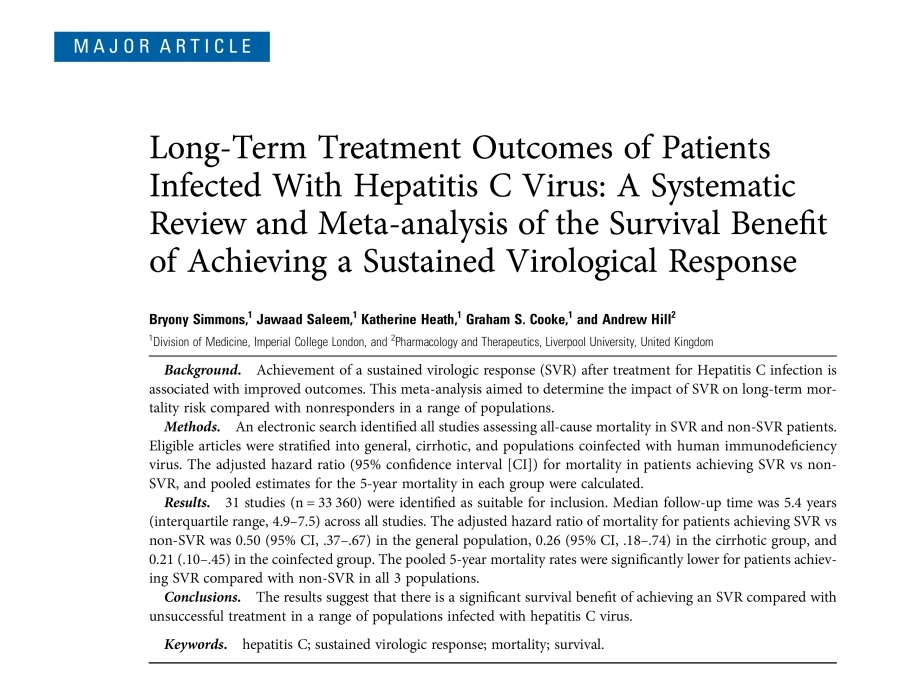
Yesterday the Guardian published an article called 'Miracle' hepatitis C drugs costing £30k per patient 'may have no clinical effect' https://www.theguardian.com/society/2017/jun/08/miracle-hepatitis-c-drugs-costing-30k-per-patient-may-have-no-clinical-effect The problem with this sensationalist headline is that while it is reporting what was said by the Cochrane Collaboration what they looked at…
There is some really interesting stuff happening in the "reverse liver fibrosis" realm. Here is state of the art in 2009 (great review of the mechanism) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702953/ where we had no clear candidates, and Here is state of the art in 2015…
Here is a copy of the generic hep c medication presentation given by Dr James Freeman at EASL 2017 today. Mostly the presentation followed the slides. The last words, with the last slide were "I want everyone to join the club, not…
“The Liver Meeting”, or AASLD 2011, was taking place at the great, glass-fronted Moscone West Convention Center in downtown San Francisco. Almost 9,000 people were there – family doctors, hepatologists, gastroenterologists, academics, drug salesmen, public health officials – all networking, trudging the…
An article with the title: Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries just made it into the Lancet print edition: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)32051-7/fulltext There is a PDF of it here
Monday, 27 June 2016 18:32
Debates in Hepatology - The use of generic medications for hepatitis C - The Case For
Written by Super User
This article about the rationale for using generic hepatitis C medication has just been published in Liver International in their section "Debates in Hepatology". James A. D. Freeman1 and Andrew Hill2 1 GP2U Telehealth, Hobart, Tas., Australia 2 St Stephens AIDS Centre, Chelsea…
Tuesday, 14 June 2016 16:39
Another US State Foots the Hepatitis C Bill - Where can you turn for therapy if you're not from Delaware?
Written by Marko
According to a report on Tuesday, said Delaware State would embark on a new Medicaid policy program all in an attempt to treat hepatitis C patients. Added pressure will not be enough Ever since Obama Administration and lawsuits assumed office, most States…
Monday, 13 June 2016 21:36
New Russian Forum for HCV - With Doctors - http://gepatitka.ru/
Written by Super User
Приветствуем, всех, кто затронут вопросами хронических гепатитов, Мы рады представить некоммерческий проект «ГЕПАТИТКА», созданный группой активистов для поддержки, помощи и общения людей, затронутых вирусными гепатитами B, C и D. Начало этого проекта было положено в марте 2012 года пациентом из Москвы во…
Monday, 30 May 2016 21:53
Getting Hepatitis C Treatment Regardless of Your Fibrosis Score
Written by Marko
In the world of Hepatitis C, the chances of getting the medical insurance to cover the high costs of your drugs comes down to a fibrosis score of an individual patient. In the US for example, Medicaid providers will in many cases…
Tagged under
Monday, 16 May 2016 16:47
CDC - Hepatitis C death rate has overtaken all other notifiable infectious condition in USA
Written by Super User
So now it's common knowledge that the Indian patent office has approved Gilead's sofosbuvir patent but.... What Happened to the Indian Official that Rejected the US Drug Company Gilead’s Patent Application in 2015? It appears that being a man of honour is…
Wednesday, 11 May 2016 16:33
India grants patent for Sovaldi - Is the Indian Hepatitis C tourism over?
Written by Marko
End of Hepatitis C Tourism? For more than a year, Hepatitis C patients who could not afford the high price tag of the novel Hepatitis C drugs in their respective countries, could go to India and buy the medicines they needed to…
Friday, 06 May 2016 19:58
Hepatitis C had become the deadliest infectious disease in the US
Written by Marko
According to the Center for Disease Control and Prevention (CDC), Hepatitis C is currently the most deadly infectious disease in the US. The findings are based on new government data about Hepatitis C related deaths in 2014. What is even more concerning…
Tagged under
Tuesday, 26 April 2016 17:35
Hepatitis C and SVR - When can you be truly sure you are Hepatitis free?
Written by Marko
Meet Steve. Steve is 58 years old and had Hepatitis C for 10 years. With all the rumors about these new direct acting antivirals (DAA) treatment being so successful, he and his doctor make a decision to start sofosbuvir-based treatment of his…
Tagged under
Saturday, 23 April 2016 21:17
REDEMPTION-1 DAA Trials for Hepatitis C presented at the International Liver Congress 2016
Written by Marko
As a part of the effort to solve the unavailability of modern Hepatitis C drugs, REDEMPTION-1 clinical trials were conducted in order to determine the safety and efficacy of generic direct acting antivirals (DAA) to treat Hepatitis C. The trials were lead by Dr.…
Tuesday, 19 April 2016 18:26
Dr. Freeman's Presentation at 2016 International Liver Congress - DAA treatment works like a charm!
Written by Marko
The International Liver Congress is a 5-day event where scientists and medical experts from all over the globe come up to speed with what is new in the latest liver research. Arguably, it is the biggest event connected with liver diseases in…
Tagged under
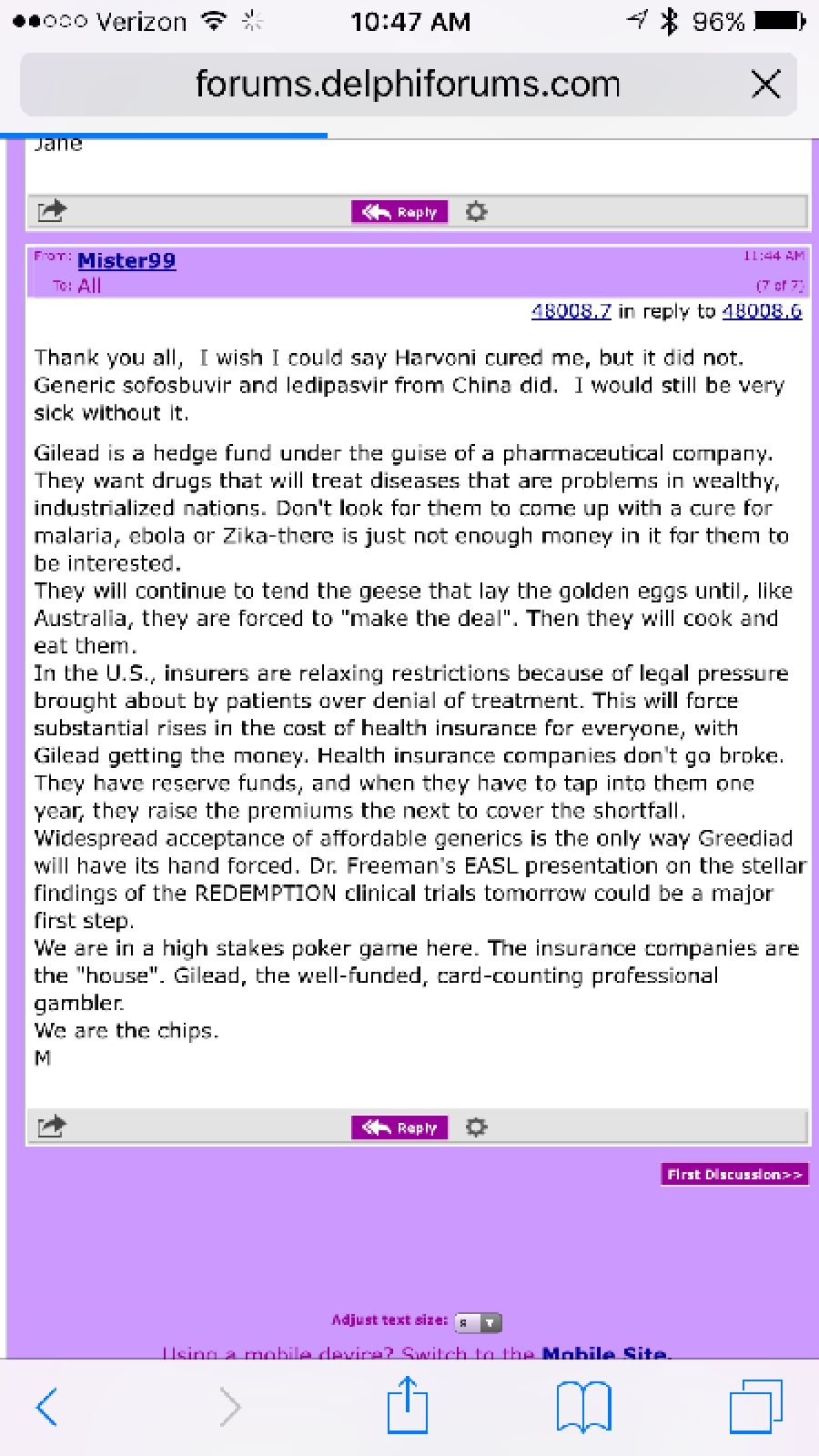
Thank you all, I wish I could say Harvoni cured me, but it did not. Generic sofosbuvir and ledipasvir from China did. I would still be very sick without it. Gilead is a hedge fund under the guise of a pharmaceutical company. They want…
Thursday, 14 April 2016 19:12
$300 Hepatitis C Cure Could be Available Within 2 Years
Written by Marko
14th April 2016 - The world renown International Liver Congress 2016 started yesterday. One of the current highlights is a study by DNDi (Drugs for Neglected Diseases initiative) which introduced a new ravidasvir and sofosbuvir combo for treating Hepatitis C. According to clinical…
Tagged under
Monday, 11 April 2016 18:49
Sovaldi was originally priced at $36,000 - Why do Hepatitis C patient have to pay more than $80,000 for it?
Written by Marko
By now pretty much everybody knows which common disease will your wallet hate the most: Hepatitis C. Let us look at a list of the most expensive drugs in the US. There are top 10 on the list; can you guess how…
Tagged under
Hepatitis C is a disease that many people have to live with for years. Even with the newly discovered treatment, some people still choose to forgo therapy due to high prices of medications. We have prepared a list of things every Hepatitis…
Tagged under
Monday, 04 April 2016 19:46
Is The Availability of Hepatitis C Drugs from India threatened?
Written by Marko
Where does India come in in the pharmaceutical industry? This is how drug industry works. New drugs are being developed by big pharmaceutical corporations in the developed world (mostly the US and Europe). When such originator drugs are launched, they are usually very…
Monday, 28 March 2016 21:07
A New Wave of Hepatitis C Patients Under the Age of 30 in the US
Written by Marko
Hepatitis C is a disease that mostly affects people after 40 years of age. Being an asymptomatic disease, Hepatitis C virus (HCV) can be hiding in the liver for more than a decade without apparent effects. This gives us an indication about…
Tagged under
More...
Friday, 25 March 2016 00:09
Example of a country where Hepatitis C spread is at an alarming rate - Pakistan
Written by Marko
We have secured a viable cure for Hepatitis C with a sofosbuvir-based regimen. However, healing the existing patients is not enough. We also have to approach systematic treatment from another angle - reduce Hepatitis C spread. In the developed world, the newly…
The Hepatitis C virus (HCV) infects people all over the world but Africa is a special case. The virus causes a chronic liver disease and is among the leading factors of liver problems in the world. The statistics from the World Health…
Tagged under
Tuesday, 15 March 2016 19:13
Hepatitis C History and Why Baby Boomers have the highest incidence of Hepatitis C infection
Written by Marko
I just opened a textbook for medical students from 1980s, doing a research on Hepatitis C. Here's the interesting thing: When looking for liver diseases, in particular Hepatitis, the table of content only lists Hepatitis A and Hepatitis B. Where is Hepatitis…
Tagged under
Thursday, 10 March 2016 20:13
Is Zepatier cost low enough to drive down the Hepatitis C treatment prices?
Written by Marko
Hepatitis C treatment prices are one of the most discussed issues in the medical world today. The patients want lower prices. The doctors have spoken against the high prices, and even the US Senate had a hearing about it. But the price of…
Tagged under