Home › Forums › Main Forum › Experts Corner › Velpatasvir – Everything you ever wanted to know and more
- This topic has 8 replies, 6 voices, and was last updated 9 years, 1 month ago by
vitrus.
-
AuthorPosts
-
8 August 2016 at 5:33 pm #21976
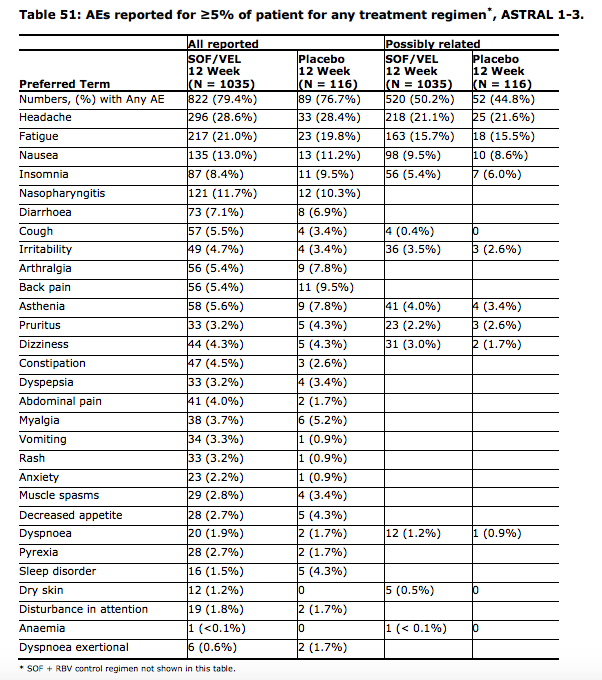
The European Medines Agency public assessment report provides some intersting ingights into Sof+Vel
p55
Velpatasvir was relatively rapidly absorbed with a tmax of 3 h after administration of the SOF/VEL FDC
tablet in healthy volunteers. The solubility is pH dependent (41 mM at pH 2 and <0.11 mM at pH 5) and
in FaSSIF and FeSSIF the solubility was <11 µM and 11 µM, respectively.Co-administration of velpatasvir with sofosbuvir has an effect on exposure of sofosbuvir (approx. 2-fold
increase).pp60
Female subjects had a lower CL/F of VEL and SOF compared to male subjects, resulting in an
approximately 50% and 20% higher exposure, respectively, compared to male subjects.pp61
The solubility of VEL is pH dependent and therefore medical products that increase gastric pH are
expected to decrease plasma concentration of VEL.pp91
YMMV
9 August 2016 at 10:43 am #22006Thanks for posting this Dr. Freeman.
A quick read shows that the addition of ribavirin (RBV) to VEL/SOF when NS5A RAVs are present yields significantly better results (GT1).
I recall reading about similar trends when RBV is added to DCV/SOF – wonder if this is correct. And I also wonder if the same applies to LDV/SOF.
The GT3 results with the addition of RBV are also interesting – even considering the small number of patients.
Page 85:
Prevalence of NS5A RAVs and Impact on Treatment Outcome
In genotype 1 HCV-infected patients, the SVR12 rates in patients with or without pre-treatment RAVs were similar in the SOF/VEL+RBV 12 Week group, in contrast to the SOF/VEL 12 and 24 Week groups, where patients with baseline RAVs had lower SVR12 rates (80% and 90%) compared to patients without RAVS (96% and 98%), respectively.
Interpretation of the results in patients with genotype 3 HCV infection is limited by the small number of patients with NS5A RAVs in each treatment group. In GT3 patients without baseline NS5A RAVs, SVR12 rates were superior in the SOF/VEL/RBV group (91%) compared to the two SOF/VEL groups (60 and 50%, in the 12 and 24 week groups, respectively).
All patients with genotype 2, 4, or 6 HCV infection achieved SVR12 irrespective of the presence of pre-treatment NS5A RAVs.
__________Have had my share of RBV to be sure – and have planned to include it yet again for retreatment. Results like this seem to bolster the decision.
J
GT 1a (~196

Diagnosed Non A/B ’85 – HCV ‘89
Rebetron INF/RBV 17 months 2000 – Failure
Infergen INF/RBV 11 months 2002 – Failure
Viekira Pak + RBV 12 weeks 2015 – Failure
VL Und at +3 weeks > EOT – EOT+12 weeks 2,240k
Resistance Tests – NS5a Q30R
SMV/DCV/SOF + RBV 24 weeks 2016
VL Det <15 +2 and +4 weeks – Und +8 weeks > EOT
SVR4, SVR12 and SVR24 Undetected9 August 2016 at 10:43 am #22007Thanks Dr Freeman,
Very heartening – particularly for us harder to treat folk. A couple of sentences for the non-experts like me sound pretty good “unprecedented SVR rates in chronic HCV infection” and “the adverse effect profile of SOF/VEL does not stand out as different from placebo”.
 10 August 2016 at 1:54 am #22028
10 August 2016 at 1:54 am #22028“Interpretation of the results in patients with genotype 3 HCV infection is limited by the small number of patients with NS5A RAVs in each treatment group. In GT3 patients without baseline NS5A RAVs, SVR12 rates were superior in the SOF/VEL/RBV group (91%) compared to the two SOF/VEL groups (60 and 50%, in the 12 and 24 week groups, respectively).”
So this is for Gt3 relapsers that have RAV?
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James10 August 2016 at 2:48 am #22029Hi Split,
Those percentages were in relation to the Astral-4 trial where all patients had decompensated cirrhosis. But the report also goes on to state:
“The remaining Achilles heel of 12 week SOF/VEL therapy concerns genotype-3 infected patients with negative predictors of cure (prior treatment experience and cirrhosis). Of note, there are limited re-treatment options for these patients, and a treatment failure in a cirrhotic patient should be considered a severe event. On the basis of available data, the CHMP recommended the addition of ribavirin in patients with compensated cirrhosis in genotype-3 infection.”
My reading of that is they are recommending addition of Riba for those with cirrhosis, relapse or both. But I’m learning on the job so run it past someone with better qualifications than me.

G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 10 August 2016 at 3:12 am #22032
10 August 2016 at 3:12 am #22032So if I add Riba to the Sof/Vel, that would push it to, what, 98%?
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James10 August 2016 at 5:14 am #22041I think it will depend on your fibrosis levels and which RAVs you have if any. How long was your previous Tx, was it just 12 weeks or some extension of that?
(Personally, while I share your enthusiasm about Velpatasvir’s potential I’m still researching my situation and gathering my thoughts at the moment and will post in my relapse thread once I have but my situation may be a little different in that I have cirrhosis and two 24 week Tx fails that included Riba already so feel the need for caution to ensure I don’t lock out future possibilities.)
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 10 August 2016 at 5:20 am #22042
10 August 2016 at 5:20 am #22042I did 16 weeks to be safe. (Sof/Dac)
I think I will order RAV and fibrosis test this time around.
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James8 December 2016 at 3:29 am #24626Thank you for information!
J. Eugene, RAVs matter mostly in those with fibrosis.
The study found that baseline RAVs significantly influence SVR in people with decompensated cirrhosis. Baseline RAVs do matter for non-cirhotic people, esp. for 3 patients (and slightly, for gen 1a). But for other genotypes (like 2,4,1b) they matter less. It’s not all black-and-white, of course, and baseline RAVs do have an influence for certain combinations of genotype and fibrosis level, but the statistics in the EMA report was for participants with decompensated cirhosis.
Gen 1b
VL pre treatment 29000 ME/ml
AST 32 ALT 94, F0
Started treatment 13 January 2017
Generic sofosbuvir/velpatasvir (Incepta)
VL 9 days into treatment <300 (undetected)
AST 13.8 ALT 22
Side effects: mild dehydration, not a problem at all if I drink water at night, nothing to worry about
Diet and gastric ph are very important with velpatasvir. One must think what and when to eat to keep gastric pH low. Side effects disappeared 2 weeks after, unless I ate anything < 4hrs before the pill. SVR60. -
AuthorPosts
- You must be logged in to reply to this topic.
