Home › Forums › Main Forum › FixHepC Admin › Q & A › G3- 16 weeks or 24 weeks?
- This topic has 11 replies, 5 voices, and was last updated 7 years, 2 months ago by
Songbird.
-
AuthorPosts
-
22 November 2018 at 4:54 pm #28728
Dear all, my mum has G3 with mild liver cirrhosis. Took Sofosbuvir+Velpatasvir+Ribavirin for 12 weeks, blood test report showed still detected but viral load was very low, it was <10. My mum still continues the medication now and planning to go for another viral load test after 16 weeks.
Question is, is it safe to stop at 16 weeks if virus is already undetected? Or should we continue the medication until 24 weeks? Hope we are able to get some help. Thank you all!22 November 2018 at 5:58 pm #28729Hello Elizabeth, welcome to the forum. Just because the test gives an untedectable result, doesn’t mean that the patient is cured and should stop the medication. The virus can come back after an undetected result if the patient doesn’t continue treatment for the duration indicated in the prescription. Also, GT3 is the hardest to treat, your mum should continue her 24 weeks treatment especially that she was detected at 12 weeks. I know that Ribavirin is not the easiest drug to take, but it will be all worth it at the end. If she continues her 24 weeks treatment, there is an excellent chance she will be cured.
Making the world a better place – one patient at a time.
22 November 2018 at 8:13 pm #28730Hi Elizabeth .
Welcome to the forum.It seems her doctor prescribed a regime a bit longer than the norm and also added Riba. (Possibly being GT 3 and chirrotic) to add some extra insurance.
Also being still <10 Det.at week 12 that added insurance could make the difference as Mar mentioned.I myself was added Riba and did extra time for the added insurance and was glad I did.
She most likely will be UND. @ 16 and hopefully the next 8 wks. after that will pass by quickly for her.
Diagnosed: 2001 GT1a , HCV since mid-70’s.
Biopsy 2010 F1
Fibroscan and Fibrosure 2018 F2Treated in trial 2010 with Dac/Peg/ Riba and Relapsed.
Resistance test 2017. Have Ns5a Rav Q30r/H58d enhanced from doing Dac.
Start Tx. Jan 18th/18 w/ Vosevi /Riba 12wks. plus 6 wks.Viekira Pak +Sof/Riba(From Dr Freeman @GP2U)
VL start: 1.6mill.ALT 125 AST 88
Wk. 4 Det @LLOQ <15.
VL Wk.8 UND Alt &Ast 22
Wk. 12 UND
EOT:UNDEOT+12 >>>UND (SVR12)! ALT11 AST13
Nov6/18 SVR 24!23 November 2018 at 3:15 am #28731Hello Elizabeth,
Without knowing the exact details it’s hard to give proper advice but…
- We know that GT3 is hardest to cure, particularly in the context of having cirrhosis
- We can observe your doctor was sufficiently concerned to add ribavirin
- We know that about 22% of patient will detected at 4 weeks, and also that these people form 44% (our data) or 50% (US VA data) of the treatment failures
- Being still detected at 12 weeks is, therefore, a worry
- The generic retreatment options for GT3 are severely limited, so
- Given we know that longer treatment is more effective than shorter treatment, if you have the ability to treat for 24 weeks, and this is affordable, and your mother is travelling OK on the treatment, I would definitely suggest you do a full 24 weeks.
PS: If you have to pay for the PCR I would suggest saving that money (it adds nothing to the cure rate) and spending it on more medication to extend the treatment duration. While showing up undetected is nice, it is pretty meaningless in terms of treatment decisions ie treat for longer or not. You mother has responded slowly so has pretty resistant virus.
YMMV
23 November 2018 at 11:32 pm #28732Doctor when you say:
“We know that about 22% of patient will detected at 4 weeks, and also that these people form 44% (our data) or 50% (US VA data) of the treatment failures
Being still detected at 12 weeks is, therefore, a worry.I had read some recent research awhile ago and actually I had seen here that you also had copied it for perusal where the conclusion now states;
“Contrary to past experience with interferon-containing treatments, low levels of quantifiable HCV RNA at [end of treatment] do not preclude treatment success,” the study authors concluded.
Also I had read this not long ago:
Conclusion:
Presence of DNQ results at EOT does not predict HCV relapse, independently to the sensitivity of the test used.
In less than half of DNQ samples, HCV-RNA detection is confirmed by the US test with LLQ of 4UI/ml.
Frequency of DNQ samples decreases during and after treatment, suggesting to reflect treatment efficacy and not aspecific RNA carry over in PCR
Am I possibly missing what the latest data seems to be saying?
Thx
S
Diagnosed: 2001 GT1a , HCV since mid-70’s.
Biopsy 2010 F1
Fibroscan and Fibrosure 2018 F2Treated in trial 2010 with Dac/Peg/ Riba and Relapsed.
Resistance test 2017. Have Ns5a Rav Q30r/H58d enhanced from doing Dac.
Start Tx. Jan 18th/18 w/ Vosevi /Riba 12wks. plus 6 wks.Viekira Pak +Sof/Riba(From Dr Freeman @GP2U)
VL start: 1.6mill.ALT 125 AST 88
Wk. 4 Det @LLOQ <15.
VL Wk.8 UND Alt &Ast 22
Wk. 12 UND
EOT:UNDEOT+12 >>>UND (SVR12)! ALT11 AST13
Nov6/18 SVR 24!24 November 2018 at 12:49 am #28733
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James24 November 2018 at 1:41 am #28735Hello Songbird,
I’m not talking about EOT results. I’m talking about on treatment kinetic decay, or more specifically rate of kinetic decay.
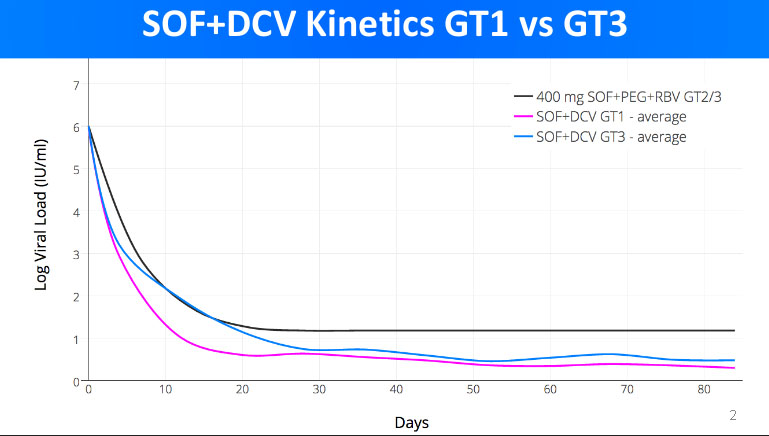
If you have a look here https://fixhepc.com/component/k2/itemlist/tag/International%20Liver%20Congress%202016.html you will see my original presentation to EASL in 2016. It contains 2 graphs about the kinetic decay of the viral load. The first is a comparator to SOF+PEG+RIBA to SOF+LDV and SOF+DCV. The
At the time I had some unique data, namely the kinetic decay for SOF+DCV in patients with both GT1 and GT3 as well as SOF+LDV for GT1. The upshot is that SOF+DCV produces a slightly faster kinetic decay than SOF+LDV in GT1 patients, and that, for SOF+DCV the GT3 kinetic decay is considerably slower than GT1.
The significance of the rate of kinetic decay is that logically we need to get to ~0-100 total virions left to get cure. This is the infective dose and also way too small to measure as you have 1000 5ml tubes of blood in you so even if the virus was completely blood born, and the cure remainder was <100 viruses only 1 in 10 blood tubes would have a single virus in it.
Anyway, it follows that if you are going down slower you need to treat for longer to have the same overtreatment insurance buffer.
YMMV
24 November 2018 at 10:26 pm #28739I was not so much referring only to EOT (however it would seem by the research I copied the conclusion seems to state that even to EOT being DNQ does not seem to be so much of a concern as it used to be.
The heading itself of the article : ” Viral load and Speed Of Decline Does Not Predict SVR”
and what is in the research that follows would seem to suggest that the speed of decline is not as prevelant and important as it used to be.
Anyway,I always appreciate your points.
S
Diagnosed: 2001 GT1a , HCV since mid-70’s.
Biopsy 2010 F1
Fibroscan and Fibrosure 2018 F2Treated in trial 2010 with Dac/Peg/ Riba and Relapsed.
Resistance test 2017. Have Ns5a Rav Q30r/H58d enhanced from doing Dac.
Start Tx. Jan 18th/18 w/ Vosevi /Riba 12wks. plus 6 wks.Viekira Pak +Sof/Riba(From Dr Freeman @GP2U)
VL start: 1.6mill.ALT 125 AST 88
Wk. 4 Det @LLOQ <15.
VL Wk.8 UND Alt &Ast 22
Wk. 12 UND
EOT:UNDEOT+12 >>>UND (SVR12)! ALT11 AST13
Nov6/18 SVR 24!25 November 2018 at 1:28 am #28740Hello Songbird,
This study was published in 2015 and the conclusions came from pooled data from small clinical trials.
Here’s a much larger VA study 4,365 patients all taking Harvoni that followed this and was published April 2016.
pp408
Significantly lower SVR rates were observed in those receiving LDV/SOF who had a 4-week on-treatment detectable HCV RNA <15 IU/mL compared to those who were undetectable by week 4.
pp410
Four-week on-treatment HCV RNA was an independent predictor of SVR when included as a variable in the models (Supporting Table S2). Having a detectable HCV RNA <15 IU/mL was associated with reduced odds of SVR compared to undetectable in the full model (OR 0.40, 95% CI 0.29-0.56, P<0.001)and in the model limited to those who completed 12weeks of treatment (OR 0.38, 95% CI 0.25-0.58, P<0.001).
pp413
In this analysis, 4‐week viral kinetics predicted SVR with a greater effect in those who received LDV/SOF. Significant reductions of 10.5% and 7.1% in SVR rates were observed in patients receiving LDV/SOF and LDV/SOF+RBV, respectively, who had a detectable 4‐week HCV RNA ≥15 IU/mL compared to those with undetectable 4‐week HCV RNA. In patients who completed 12‐week courses of LDV/SOV there remained a significant 6.4% reduction in SVR rates between those with detectable 4‐week HCV RNA ≥15 IU/mL and those with undetectable 4‐week HCV. For those who completed 12 weeks of LDV/SOF+RBV the difference in SVR rates was no longer statistically significant. In multivariable analysis, having a HCV RNA ≥15 IU/mL after 4 weeks of treatment was associated with a 60% reduced odds of achieving SVR for the cohort and a 62% reduced odds of achieving SVR for those who completed 12 weeks of treatment. The VA guidance recommends obtaining 4‐week HCV RNA testing, thus providing a large sample size to evaluate this variable which has not generally been assessed in prior LDV/SOF studies. The clinical implications of this finding on treatment decisions, such as potentially adding RBV or extending treatment duration based on 4‐week on‐treatment HCV RNA, warrant further study.
Call it an inconvenient truth if you like, but a truth it is. I have 3460 patients at last count and initial slow responders used to feature disproportionately in the retreatment group. They feature less now because I extend or modify their treatment.
You may find this interesting in terms of digging into the science of what needs to be done to get to cure:
https://www.bhiva.org/file/lOvBacnRQiHzM/ValeriaCento.pdf
You may also note that this is an early study based on small numbers and concludes in a 1/2 and 1/2 way saying
- In contrast with IFN-containing regimens, a Rapid Viral Response after 4 weeks of INF-free treatment seems not to correlate with SVR12 (proven wrong by VA)
- Cirrhotic patients have a slower HCV-RNA kinetics, and in this population response-guided therapy can still have a role in determining the optimal duration of treatment. (right)
YMMV
25 November 2018 at 9:25 pm #28742I always appreciate the copy of that data to look over as I find it interesting the mechanism of the life-cycle and in turn the inhibitors ability to interupt such.
I guess it is just my inquisitve nature, as I have worked in data research for the better part of 35 years.(not HCV).
This particular pooled -data is “current” and ” Not” a small number of patients .(approx.1300)
I found it very interesting that it states that all the relaspers were indeed UND. @week4 and that many of the ones that went on to SVR were UND. much later
Granted this is study was done on Mavyret only.
As seen ,the time to viral suppression was looked at by a number of factors(ie. GT, BMI, PPI use, Fibrosis Stage and RAS).
So ,it would seem (to me anyway) that they are looking at more specific reasons and what factors go into the speed of decline and what at the end of the day that means in terms of SVR, rather than just a broad based approch of “extend if there is late decline”
Again thanks,appreciate it.
(http://www.natap.org/2018/IDWeek/IDWeek_38.htm
Diagnosed: 2001 GT1a , HCV since mid-70’s.
Biopsy 2010 F1
Fibroscan and Fibrosure 2018 F2Treated in trial 2010 with Dac/Peg/ Riba and Relapsed.
Resistance test 2017. Have Ns5a Rav Q30r/H58d enhanced from doing Dac.
Start Tx. Jan 18th/18 w/ Vosevi /Riba 12wks. plus 6 wks.Viekira Pak +Sof/Riba(From Dr Freeman @GP2U)
VL start: 1.6mill.ALT 125 AST 88
Wk. 4 Det @LLOQ <15.
VL Wk.8 UND Alt &Ast 22
Wk. 12 UND
EOT:UNDEOT+12 >>>UND (SVR12)! ALT11 AST13
Nov6/18 SVR 24!26 November 2018 at 4:43 am #28743Hello Songbird,
You can’t really compare Maviret to Sofosbuvir based regimens and say what is true for the most potent NS3/4A and NS5A agents ever invented is applies to NS5A/NS5B combinations.
HCV represents a heterogeneous quasispecies – the population in any patient exhibits a great deal of variability. It stands to reason some is easy to kill (call them ordinary infantry), some is harder to kill (call them special forces with body armour) and some is harder again to kill (call them tankers).
Rapid viral suppression represents two distinct things. First we are looking at the decline of the easy to kill population (and this is relatively irrelevant) but secondly, and perhaps more importantly, it represents the window period where there are viable replicons and a drug-driven selective pressure on resistance. In the context of drugs being present, we are selecting for the resistant forms to replicate, and one more mutation could be enough to overcome the EC50 of the drugs we are using.
We do know from HIV that continuing to use drugs where the patient has a viral load leads to the development of resistance.
We are lucky that the drugs work as well as they do.
To me, the issue with fibrosis is that the lower blood supply of fibrotic tissue leads to lower tissue concentrations and thus the potential for the drugs to be present at < EC50. The same goes for BMI - we have certainly seen Daclatasvir toxicity in < 50kg patients and treatment failure more common in > 150kg patients. This is a problem with the one size dose fits all model currently in use. Gilead’s research in children adjusts the dose down based on bodyweight (makes perfect sense) but there is no dose adjustment UP for larger people. There is dose adjustment up for many other drugs – antibiotics, anaesthetics, …
YMMV
26 November 2018 at 6:00 am #28744Thanks Dr.Always appreciate your insight.
Diagnosed: 2001 GT1a , HCV since mid-70’s.
Biopsy 2010 F1
Fibroscan and Fibrosure 2018 F2Treated in trial 2010 with Dac/Peg/ Riba and Relapsed.
Resistance test 2017. Have Ns5a Rav Q30r/H58d enhanced from doing Dac.
Start Tx. Jan 18th/18 w/ Vosevi /Riba 12wks. plus 6 wks.Viekira Pak +Sof/Riba(From Dr Freeman @GP2U)
VL start: 1.6mill.ALT 125 AST 88
Wk. 4 Det @LLOQ <15.
VL Wk.8 UND Alt &Ast 22
Wk. 12 UND
EOT:UNDEOT+12 >>>UND (SVR12)! ALT11 AST13
Nov6/18 SVR 24! -
AuthorPosts
- You must be logged in to reply to this topic.
