Home › Forums › Main Forum › Experts Corner › Retreatment Corner › Managing Patients After DAA Treatment Failure
- This topic has 19 replies, 12 voices, and was last updated 9 years, 7 months ago by
 Dr James.
Dr James.
-
AuthorPosts
-
1 March 2016 at 3:45 pm #13027
Any SVR rate <100% means a small number of people will not attain SVR. Don't panic we are currently running 95.1% SVR but that still means for every 100 people on generic treatment 5 will not SVR.
I came across an excellent article from Dr Jordan Feld here:
He was kind enough to allow it to be reprinted here.
How I Manage Patients With HCV After DAA Treatment Failure
Jordan J. Feld, MD, MPH – 19/1/2016
The remarkable success of the new direct-acting antiviral (DAA) therapies for chronic HCV infection has led many patients and physicians to expect a cure whenever these agents are used. Although this is indeed the outcome for most, what can we offer the few patients in whom these treatments fail?
The data on retreatment are just starting to emerge. Whereas these studies cannot address every situation, a few general approaches are supported both by good logic and at least some empirical evidence: 1) switching to a different DAA class or classes, 2) treating for longer, and 3) adding ribavirin.
Treating With a Different DAA Class
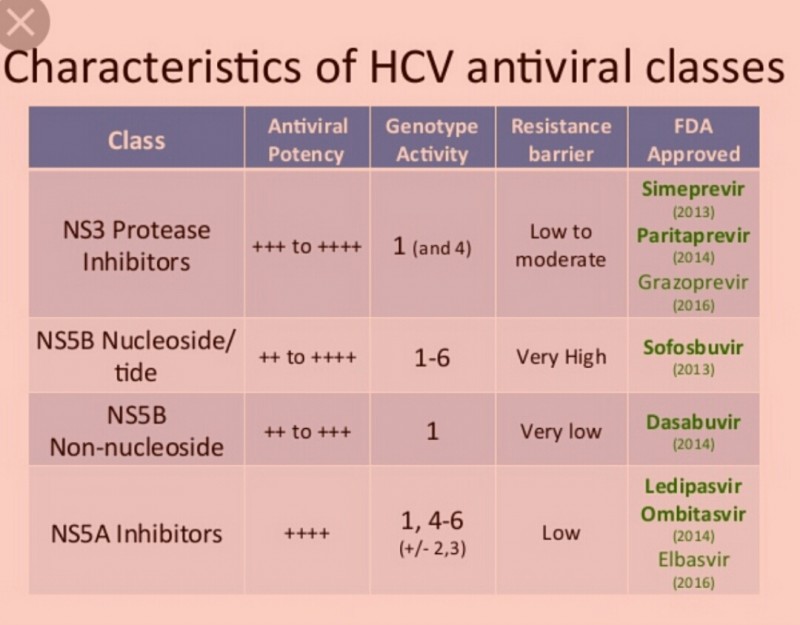
The simple approach of switching DAA classes is an obvious option for retreatment. It is certainly effective as there is no cross-resistance across DAA classes. This was established by the outstanding results of clinical trials in which patients who experienced protease inhibitor (telaprevir or boceprevir) failure were retreated with combinations of a nucleotide polymerase inhibitor (sofosbuvir) and an NS5A inhibitor (daclatasvir or ledipasvir).
However, switching to a different DAA class is not always possible, owing to contraindications, comorbidities, or potential drug–drug interactions, and may not be absolutely necessary if the other approaches are followed.
The biggest challenge in treating with a different class is NS5A resistance. NS5A resistance associated variants (RAVs) are very fit; they often emerge even before treatment and almost always persist in patients for whom an NS5A-containing regimen fails. Since NS5A inhibitors are part of most approved and investigational regimens, it can be difficult to avoid NS5A inhibitors when retreating.
Extending Treatment Duration
Retreating patients with a longer duration of the same treatment that failed can help many patients, but it may not be enough to overcome RAVs. This was demonstrated in the initial retreatment trial presented at EASL 2015 by Lawitz and colleagues. Patients for whom 8 or 12 weeks of ledipasvir/sofosbuvir failed were retreated with ledipasvir/sofosbuvir again—this time for 24 weeks. Among patients without NS5A RAVs, this approach worked very well, resulting in an SVR12 rate of 100%. However, among those with NS5A RAVs, this strategy was suboptimal, with an SVR12 rate of only 60%. Even more concerning, 4 of the patients who failed the second course of therapy also developed RAVs to sofosbuvir. Fortunately, lessons were learned and the retreatment trials presented at AASLD this year proved much more successful.
Adding Ribavirin
Data from the 2015 AASLD annual meeting highlight that retreatment with a previously unsuccessful regimen is possible with the help of ribavirin. In the C-SWIFT trial, patients who had relapsed after 4-8 weeks of treatment with grazoprevir (a protease inhibitor), elbasvir (an NS5A inhibitor), and sofosbuvir (an NS5B inhibitor) were retreated with the same combination of DAAs but for longer (12 weeks) and with the addition of ribavirin. With this approach, all 23 patients achieved SVR12.
The benefit of adding ribavirin to retreatment is not exclusive to NS5A inhibitors. In a small study of patients for whom a protease inhibitor regimen had failed, most patients were retreated with a protease inhibitor–containing regimen (ombitasvir/paritaprevir/ritonavir plus dasabuvir) plus sofosbuvir and ribavirin. Fourteen of these 15 patients achieved SVR12.
Although the mechanism(s) of action of ribavirin remain elusive, almost all studies using ribavirin with DAAs have shown that it delays or prevents the emergence of resistance, particularly in difficult-to-cure populations. Because ribavirin seems to raise the barrier to resistance, particularly for low-barrier DAAs including NS5A and protease inhibitors, it makes sense to add ribavirin to DAAs when retreating patients with presumed or known resistance.
Combining Strategies
All of the small retreatment studies I’ve mentioned successfully combined at least 2 of 3 approaches: switching to a different DAA class, treating for longer, or adding ribavirin. However, most of these retreatment studies were in patients without cirrhosis, a relatively easy-to-cure population. In clinical practice, many of the patients whose first treatment fails are those with advanced cirrhosis. For these tougher-to-treat patients, a combination of all 3 approaches would likely be worthwhile.
Consider Waiting to Retreat
Although retreatment strategies are becoming more clearly defined, it is also important to consider that, for most patients, retreatment is not an emergency. For all patients except those with life-threatening cryoglobulinemic vasculitis, retreatment can almost certainly wait. Even for patients with advanced cirrhosis, waiting is not always a bad decision; SVR does not reverse liver failure in many patients, so those who cannot wait may actually be better served by being listed for a transplant.
There are at least 2 advantages to exercising a bit of patience. First, unfit RAVs, such as those to sofosbuvir, often disappear with time. Of more importance, research in HCV management continues to move at breakneck speed. The next meeting in this field may well offer the data we need to guide our decisions so we avoid failing a second time.
YMMV
31 May 2016 at 1:19 pm #18113The benefit of adding ribavirin to retreatment is not exclusive to NS5A inhibitors. In a small study of patients for whom a protease inhibitor regimen had failed, most patients were retreated with a protease inhibitor–containing regimen (ombitasvir/paritaprevir/ritonavir plus dasabuvir) plus sofosbuvir and ribavirin. Fourteen of these 15 patients achieved SVR12.
Am I reading that correctly? They used two different NS5B inhibitors together? (Sofosbuvir and Dasabuvir)
31 May 2016 at 7:07 pm #1814331 May 2016 at 7:22 pm #18144The above re-treatment combo is not written very clearly. I would read
(ombitasvir/paritaprevir/ritonavir plus dasabuvir) plus sofosbuvir and ribavirin
to mean
(ombitasvir (NS5A) OR paritprevir (NS3/NS4A) OR ritonavir (HIV protease))
PLUS
dasabuvir (NS5
PLUS
sofosbuvir (NS5
PLUS
ribavarinThis makes two NS5B inhibitors.
Diagnosed Jan 2015: GT3, A0+F0/F1. Fatigue + Brain-Fog.
Started Sof+Dac from fixHepC 10-Nov-2015. NO sides.
Pre-Tx: AST 82, ALT 133, Viral Load 1 900 000.
Week4: AST 47, ALT 58. VL < 15 (unquantifiable). Week12 (EOT): AST 30, ALT 26, VL UND Week16 (EOT+4): AST 32, ALT 28, GGT 24, VL UND Week28 (EOT+16): AST 26, ALT 22, GGT 24, VL UND Ever grateful to Dr James. Relapsed somewhere after all that... Bummer! Jan 2018: VL 63 000 (still GT3).31 May 2016 at 7:47 pm #18145Per the indisputable authority of Wikipedia



Dasabuvir acts as a NS5B (an RNA-directed RNA polymerase) inhibitor.
and
Sofosbuvir is a prodrug using the ProTide biotechnology strategy. It is metabolized to the active antiviral agent 2′-deoxy-2′-α-fluoro-β-C-methyluridine-5′-triphosphate. The triphosphate serves as a defective substrate for the NS5B protein, which is the viral RNA polymerase, thus acts as an inhibitor of viral RNA synthesis.But maybe there’s something here I’m missing.
Viekira Pak is two pills … a combo pill of Ombitasvir/Paritaprevir/Ritonavir and another pill of only Dasabuvir.
31 May 2016 at 8:54 pm #18146klhilde wrote:The benefit of adding ribavirin to retreatment is not exclusive to NS5A inhibitors. In a small study of patients for whom a protease inhibitor regimen had failed, most patients were retreated with a protease inhibitor–containing regimen (ombitasvir/paritaprevir/ritonavir plus dasabuvir) plus sofosbuvir and ribavirin. Fourteen of these 15 patients achieved SVR12.
Am I reading that correctly? They used two different NS5B inhibitors together? (Sofosbuvir and Dasabuvir)
Yes – I believe you are correct – in part. Sofosbuvir is characterized as a nucleotide polymerase inhibitor.
This looks like the AbbVie Quartz-1 study where they added Sofosbuvir to their existing Viekira Pak plus RBV regiment (12 weeks).
Some of the details here:
Note the size of the study group – it is indeed very small.
J
GT 1a (~196

Diagnosed Non A/B ’85 – HCV ‘89
Rebetron INF/RBV 17 months 2000 – Failure
Infergen INF/RBV 11 months 2002 – Failure
Viekira Pak + RBV 12 weeks 2015 – Failure
VL Und at +3 weeks > EOT – EOT+12 weeks 2,240k
Resistance Tests – NS5a Q30R
SMV/DCV/SOF + RBV 24 weeks 2016
VL Det <15 +2 and +4 weeks – Und +8 weeks > EOT
SVR4, SVR12 and SVR24 Undetected31 May 2016 at 9:56 pm #18147Thanks, I stand corrected. Sofosbuvir is a polymerase inhibitor, and NS5B is the polymerase in HCV.
https://en.wikipedia.org/wiki/NS5B
Diagnosed Jan 2015: GT3, A0+F0/F1. Fatigue + Brain-Fog.
Started Sof+Dac from fixHepC 10-Nov-2015. NO sides.
Pre-Tx: AST 82, ALT 133, Viral Load 1 900 000.
Week4: AST 47, ALT 58. VL < 15 (unquantifiable). Week12 (EOT): AST 30, ALT 26, VL UND Week16 (EOT+4): AST 32, ALT 28, GGT 24, VL UND Week28 (EOT+16): AST 26, ALT 22, GGT 24, VL UND Ever grateful to Dr James. Relapsed somewhere after all that... Bummer! Jan 2018: VL 63 000 (still GT3).31 May 2016 at 11:05 pm #18149J Eugene, I’ve read your signature, so I understand why you’re on top of this info.
I don’t know where you’re from, or whether you have a retreatment plan put together yet, but did you see what Dr. Freeman said in this thread? Might want to keep that little bit of special info in mind.
2 June 2016 at 7:58 am #18247Same same but different
Attachments:18 July 2016 at 2:13 pm #21028”James-Freeman-facebook” wrote:Any SVR rate <100% means a small number of people will not attain SVR. Don't panic we are currently running 95.1% SVR but that still means for every 100 people on generic treatment 5 will not SVR.
I came across an excellent article from Dr Jordan Feld here:
Consider Waiting to Retreat
Although retreatment strategies are becoming more clearly defined, it is also important to consider that, for most patients, retreatment is not an emergency. For all patients except those with life-threatening cryoglobulinemic vasculitis, retreatment can almost certainly wait. Even for patients with advanced cirrhosis, waiting is not always a bad decision; SVR does not reverse liver failure in many patients, so those who cannot wait may actually be better served by being listed for a transplant.
There are at least 2 advantages to exercising a bit of patience. First, unfit RAVs, such as those to sofosbuvir, often disappear with time. Of more importance, research in HCV management continues to move at breakneck speed. The next meeting in this field may well offer the data we need to guide our decisions so we avoid failing a second time.
I seemed to have developed a sudden interest in this thread and was wondering in regard to the bold bit above – does this mean if left alone, there is some chance the RAV in-breads may be that inbred, they simply cark it and die out. Can’t find any articles along this line though.
J.
18 July 2016 at 5:46 pm #21032Hello sabrecat,
Sorry you need to be interested.
You must be F4 to have had an HCC so I presume your sig F3a means GT3a
In the near term Sof+Dac +/- Riba for 24 weeks is as good as it gets although PEG remains quite functional in GT3 for people known to be responders.
Next week Sof+Vel +/- Riba may well appear. GS-9857 is still a few months away.
With HIV resistance increase if replication is happening so suppressing replication is important on treatment. If you did not break through Sof should still work fine.
YMMV
18 July 2016 at 10:18 pm #21034Someone please advise.
Geno 1. Infected for 30+ years.Treated in 2003-2004 with ribivirin-interferon – 48 weeks. Didn’t take.
Applied for and was denied Harvoni by insurance. Rather than wait, I went directly to China (Mesochem) and received generic Harvoni.
Got a local druggist to compound for me and treated.
At 6 weeks was undetected. Just did my blood work at 24 weeks and:
RDW-CV High
Neutrophils – Low
Lymphocytes – High
Monocytes High
ALT – High (7
HCV IU/mL – 1098407
HCV LOG IU/ml – 6.04Did I really not clear???
Please talk me off the ledge.
18 July 2016 at 11:57 pm #21036Damn it! Tough news Cruzan. Relapse/failure is something I’ve dealt with, and I know how gut wrenching it is. Hang in. Someone who has a much better handle on this than I will be along soon.
My thoughts and prayers are with you in this.
19 July 2016 at 6:24 am #21048I am also gut punched by your news, but I know your in the right place and something will come more sooner than later. Stay the course, we’ll be here with you.
Contracted HCV 1980’s
Geno Type 1a
F3 ( doc says once treated I’ll be F2 maybe F1)
Meds shipped 6/17/2016 arrived early 7/2016Viral count – 3,471,080
4 week quantitative bloods: August 17, 2016. I have been diagnosed as <15 (told undetected)
8 week quantitative bloods: September 14th. I have been diagnosed as <15 (told undetected)
11 week PCR RNA Qualitative bloods: September 26th 2016 – Undetected
December 19th 2016: Cured!
Viral count: zero!!!
2018 viral count: still zero!
Cured!19 July 2016 at 7:32 am #21052Hi Cruzan,
Sorry to hear your news. Unfortunately even with these new drugs the results are still only about 95% successful overall meaning not all of us manage to acheive SVR on our first attempt. The good news is that for those of us in that position the science and R&D is progressing fairly quickly these days so there are further options available either now or in the very near future depending on our individual situation.
Having recently found myself in a similar situation I would suggest that your best option is to get all your information and results together and have a discussion with someone who understands the HCV retreatment process. One option that I can recommend for that would be a ‘virtual’ consultation over Skype or similar with https://gp2u.com.au who will be able to provide you with good advice as to your various options for the future whether that be retreatment or otherwise.
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND



-
AuthorPosts
- You must be logged in to reply to this topic.