Home › Forums › Main Forum › Media & News › Sofosbuvir-Velpatasvir – suits all genotypes
- This topic has 8 replies, 7 voices, and was last updated 10 years, 3 months ago by
 Dr James.
Dr James.
-
AuthorPosts
-
17 November 2015 at 9:34 am #4097
http://www.sciencedaily.com/releases/2015/11/151116181750.htm
“Current approved treatments for chronic HCV are not equally effective in combating the virus’ different genotypes. Testing to determine the genotype and subtype of the virus is required before treatment could be initiated. But the combination of sofosbuvir-velpatasvir has been shown to be applicable to all strains of HCV, effectively eliminating the need to test for the viral genotype — an obstacle that often delayed treatment.
The regimen was tested in an international, randomized, double-blind placebo-controlled phase three trial conducted at 81 sites in eight different countries. After 12 weeks, 99 per cent of the 624 patients who had been treated with a daily tablet of sofosbuvir-velpatasvir experienced a sustained virologic response — the medical term for eradication or cure of HCV — meaning that patients remained free of the virus three months after completing treatment. None of the 116 patients receiving a placebo experienced the same result.”
17 November 2015 at 9:43 am #4099Gilead’s new possible game-changer…applicable to all genotypes except 3
GT1a since 1988, diagnosed 1990
F0, tx naive
VL 262,000 ALT 40 AST 26 GGT 13 Fibroscan 04/12/15 – 2.9
Started Mesochem sof/dac 12 weeks 01/01/2016
11/02/2016 – 6 weeks UNDETECTED
AST 26
ALT 2617 November 2015 at 9:50 am #4100You’re right – no 3, I missed that (brain fog). I didn’t even know there was a 5 & 6.
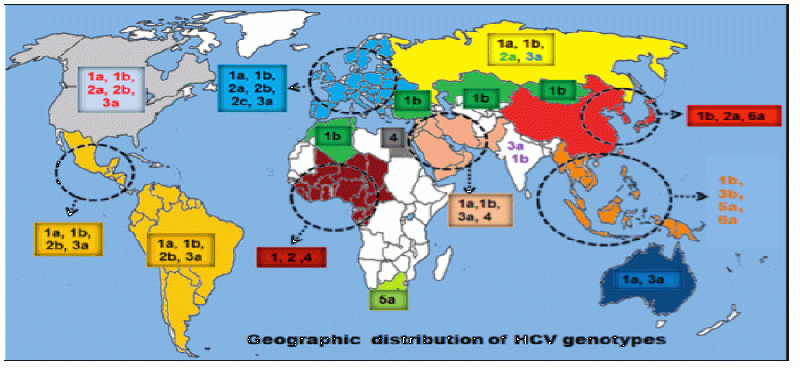
17 November 2015 at 11:19 am #4102Yep they are pretty confined to parts of Asia/S Africa
GT1a since 1988, diagnosed 1990
F0, tx naive
VL 262,000 ALT 40 AST 26 GGT 13 Fibroscan 04/12/15 – 2.9
Started Mesochem sof/dac 12 weeks 01/01/2016
11/02/2016 – 6 weeks UNDETECTED
AST 26
ALT 2621 November 2015 at 1:43 am #4305I’m not convinced it’s better than Dac.
21 November 2015 at 2:04 am #4307klhilde wrote:I’m not convinced it’s better than Dac.
Me either in fact i’m not convinced that Led is better than Dac in Gen1 patients either. Gilead makes both products so Sof Led was offered more I think trying to cut BMS out.
Two time relapser.
SVR 4 achieved 12/16 at last
SVR 12 achieved 22/02/2017 The Bastard has been defeated


GT 3 – about 28 yrs with HCV
21 November 2015 at 2:15 am #4312We have to take into account Gilead’s dishonesty in the way it presents it’s trial results.
And, remember, Gilead bought the company that created Sofosbuvir. They then quietly changed the product, making it slightly inferior but giving it a better shelf life, after trialling it. They then created their own NS5A inhibitor, Ledipasvir. It has a lower kill log than Daclatasvir, but Gilead’s power and persuasion have made it’s combination with Sovaldi (Harvoni) the market leader and preferred medicine for many heppers.
Frankly, if a Gilead exec shook my hand (unlikely, I know), I’d check how many fingers I had left.
21 November 2015 at 2:18 am #431399% SVR 12 for genotype 2 in study for sovaldi and velpatasvir this says:
GT 2b; since 80’s, no prior tx, sofosbuvir and daclatasvir compounded from API’s at Kingswood Pharmacy in Sydney, started tx nov 6,2015, undetected at 4 wks, UND at 8 weeks, UND at 1 week after EOT, UND at 4 weeks after EOT and UND at 8 weeks after EOT. I feel GOOD!! I knew that I WOULD!””
21 November 2015 at 3:02 am #4319So if you read the study http://www.healio.com/hepatology/hepatitis-c/news/online/%7B223dc0cb-9261-4e9c-a7d5-42ed1b96d88e%7D/astral-2-sovaldivelpatasvir-bests-standard-therapy-in-hcv-genotype-2
There were 134 patients in the sofosbuvir/velpatasvir….
First consider margin of error https://www.surveymonkey.com/mp/margin-of-error-calculator/
If you use a conservative 1,000,000 for the population size, a 95% confidence interval and 134 sample you will see the margin of error is 9%
So this could quite possibly represent a 90% real cure rate.
99% is interesting because 1 patient cure either way at this study size make big differences
133/134 = 99.2% (rounds to 99%)
132/134 = 98.507% (rounds to 99%)
131/134 = 97.76% (rounds to 98%)There were 134 patients in the sofosbuvir/velpatasvir arm and 132 in the sofosbuvir/ribavirin arm. One patient in the velpatasvir arm stopped treatment due to an adverse event on day 1 and one patient in the ribavirin arm was lost to follow-up after week 10.
There were two deaths in the velpatasvir group, but neither was determined to be related to the study regimen.
Here is better data reported in the mainstream literature:
http://www.ncbi.nlm.nih.gov/pubmed/26569658
Results Of the 267 patients who received treatment, 78% had HCV genotype 1, 4% genotype 2, 15% genotype 3, 3% genotype 4, and less than 1% genotype 6; no patients had genotype 5. Overall rates of sustained virologic response were:
- 83% (95% confidence interval [CI], 74 to 90) among patients who received 12 weeks of sofosbuvir-velpatasvir,
- 94% (95% CI, 87 to 9
 among those who received 12 weeks of sofosbuvir-velpatasvir plus ribavirin, and
among those who received 12 weeks of sofosbuvir-velpatasvir plus ribavirin, and - 86% (95% CI, 77 to 92) among those who received 24 weeks of sofosbuvir-velpatasvir.
And these results are also worth looking at (lots of < 90% SVR results in there): http://www.ncbi.nlm.nih.gov/pubmed/26551051
So in bigger studies it does not look superior to Daclatasvir.....
YMMV
-
AuthorPosts
- You must be logged in to reply to this topic.
