Home › Forums › Main Forum › Patient Stories › Relapse Corner – Next Steps › Voxilaprevir (akaGS-9857) – A new Protease Inhibitor
- This topic has 49 replies, 11 voices, and was last updated 8 years, 7 months ago by
 Dr James.
Dr James.
-
AuthorPosts
-
25 February 2017 at 3:47 am #25400
Maybe. At any rate, good news as it means Vox is coming soon……..
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James25 February 2017 at 4:34 am #25401splitdog wrote:A Voxilaprevir intermediate is now available in China.

 Thanks for finding this SD, although an intermediate won’t be suitable for direct purchase and compounding due to solubility issues/bioavailability it means that generics manufacturers are able to develop a usable final product.
Thanks for finding this SD, although an intermediate won’t be suitable for direct purchase and compounding due to solubility issues/bioavailability it means that generics manufacturers are able to develop a usable final product. 
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 25 February 2017 at 5:12 am #25402
25 February 2017 at 5:12 am #25402I did not understand that an intermediate is not a good product.
The bottom line, American manufacturers are sourcing API’s in the 3rd world. I was cured by Incepta Twinvir that is produced with Chinese API’s, perhaps even an exact copy of Harvoni. The generics are the same meds as the name brand. You guys are going to be cured…..and will join me in giving the middle finger daily to the companies like Gilead.
25 February 2017 at 12:47 pm #25403Greedfighter, it means intermediate is for factories, home compounding is not possible, unlike with some other drugs.
The quality of drug depends on quality control and chemical processes employed by both ingredient and pill manufacturer. Every factory is different, but both Bangladeshi manufacturers have been successful so far at ensuring the quality of the final product.Gilead produces api in factories in many countries, the data is publicly available.
Gen 1b
VL pre treatment 29000 ME/ml
AST 32 ALT 94, F0
Started treatment 13 January 2017
Generic sofosbuvir/velpatasvir (Incepta)
VL 9 days into treatment <300 (undetected)
AST 13.8 ALT 22
Side effects: mild dehydration, not a problem at all if I drink water at night, nothing to worry about
Diet and gastric ph are very important with velpatasvir. One must think what and when to eat to keep gastric pH low. Side effects disappeared 2 weeks after, unless I ate anything < 4hrs before the pill. SVR60.25 May 2017 at 10:03 pm #26177Curious to know of any updates on the general availability of voxilaprevir – or any of the other second generation NS3/4A protease inhibitors.
Gilead submitted their three-drug combo (voxilaprevir/velpatasvir/sofosbuvir) for FDA approval here last December – have not read of their progress.
Are the manufacturers of generic medicines moving closer to the release of NS3/4A protease inhibitors ??
GT 1a (~196

Diagnosed Non A/B ’85 – HCV ‘89
Rebetron INF/RBV 17 months 2000 – Failure
Infergen INF/RBV 11 months 2002 – Failure
Viekira Pak + RBV 12 weeks 2015 – Failure
VL Und at +3 weeks > EOT – EOT+12 weeks 2,240k
Resistance Tests – NS5a Q30R
SMV/DCV/SOF + RBV 24 weeks 2016
VL Det <15 +2 and +4 weeks – Und +8 weeks > EOT
SVR4, SVR12 and SVR24 Undetected26 May 2017 at 10:47 am #26185Hello J. Eugene,
I know at least one of the generics manufacturers are looking at it. FDA approval has to happen first but it will probably go down as observed before with Bangladesh first and India 6-9 months after FDA approval.
We do have a very good NS3/4A and NS5A combination now – Viekira.
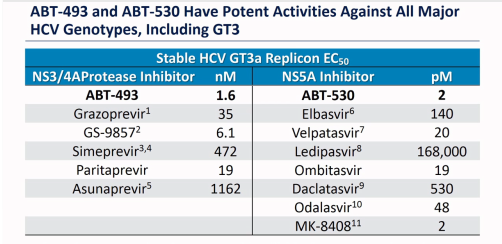
As you can see, in GT3 paritaprevir is almost as good as Vox (GS-9857) and better than the Elbasvir in Zepatier (which is approved for GT3 with Sof). The ombitasvir is just as potent as Velpatasvir and stronger than the Grazoprevir in Zepatier.
On the practical side of things, Abbvie Viekira is marketed in Egypt and can be sourced reasonable affordably, meaning a Sof+Vel+Vox potency combination is available now.
The trials of Sof + Viekira and Sof + Zepatier show it works well:
YMMV
6 June 2017 at 12:11 am #26324I thought the US FDA had established fast-track on pending Voxilaprevir approval. That means within 6 months. Tomorrow is 6 months. No word yet?
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James6 June 2017 at 8:03 am #26328Hello Splitdog,
It’s not scheduled for approval until August 8 2017

YMMV
6 June 2017 at 10:58 pm #26339Thank you Dr. Freeman. I was going to say the wait is killing me…..but no.

How long after that do you think it will be before Beacon is ready to dispense Vox?
My Vel expiration date is Jan 2018.
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James7 June 2017 at 12:56 pm #26348Hello Splitdog,
It will definitely be after the FDA approval, but how fast depends on things that have not happened yet. They were pretty quick last time, but lots of things have to happen internally before they can release it.
YMMV
24 June 2017 at 10:02 am #26497Some progress – and a name for Gilead’s new 3DAA
Vosevi
Gilead Press Release – June 23, 2017
European CHMP Adopts Positive Opinion for Gilead’s Vosevi® (Sofosbuvir/Velpatasvir/Voxilaprevir) for the Treatment of All Chronic Hepatitis C Genotypes
Not certain how long the European Commission will take to approve the drug.
The FDA here has a target action date under the Prescription Drug User Fee Act of August 8, 2017.
It will be very interesting to see how Gilead prices Vosevi and how it is marketed alongside their existing Epclusa (Sofosbuvir/Velpatasvir) and Harvoni (Sofosbuvir/Ledipasvir)
Will be watching – and watching too for the generic counterparts
J
GT 1a (~196

Diagnosed Non A/B ’85 – HCV ‘89
Rebetron INF/RBV 17 months 2000 – Failure
Infergen INF/RBV 11 months 2002 – Failure
Viekira Pak + RBV 12 weeks 2015 – Failure
VL Und at +3 weeks > EOT – EOT+12 weeks 2,240k
Resistance Tests – NS5a Q30R
SMV/DCV/SOF + RBV 24 weeks 2016
VL Det <15 +2 and +4 weeks – Und +8 weeks > EOT
SVR4, SVR12 and SVR24 Undetected15 July 2017 at 8:10 am #26582Any day now………….

Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James16 July 2017 at 11:48 am #265898th August for the Gilead product Splitdog
YMMV
17 July 2017 at 3:46 pm #26594So …
FDA approval date will be August 8 …
… and …
Alibaba already shows a trading company, Zhejiang Chemicals, listing three different intermediates …Therefore …
Beacon should have Magicavir* available on August 10.
(Okay, maybe not quite that fast.)Recent history suggests it won’t be long now Splitdog. I bet Dr. Freeman actual has a pretty good idea of when it will be. In any case, we’re cheering for you Splitdog.
_______________* my entry in the naming contest.
_______________I’ve been curious about the nomenclature, and the obvious just struck me ….
NS5B RNA polymerase inhibitors …. the “B” = buvir
NS5A inhibitors … the “A” = asvir
NS3/4A protease inhibitors … the “P” in Protease = previr18 July 2017 at 3:40 am #26595Thank you! I can hardly wait now……..

Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James -
AuthorPosts
- You must be logged in to reply to this topic.
