Home › Forums › Main Forum › Genotype Specific › Genotype 1 (54%) › Which medication ?
- This topic has 7 replies, 5 voices, and was last updated 7 years, 9 months ago by
 Gaj.
Gaj.
-
AuthorPosts
-
12 August 2017 at 9:29 pm #26730
I would go with Velpatasvir/sobosbuvir. (Epclusa-Sofosvel) Slightly better than Harvoni. But in your case, (low VL-treatment naive), Harvoni should work just fine. Ask the doctor. He should be here soon…… Good luck!
Genotype 3
VL 4,100,000
ALT 101 AST 71
Treatment Naive
Started Sof/Dac Jan 12, 2016
VL= <15 4 weeks in. AST/ALT normal.
VL=UNDETECTED 8 weeks in.
SVR4= Virus back. 3,300,000Started generic Epclusa Sep. 23, 2017
4 weeks in <15 *Detected.
12 weeks in <15 *Not Detected.
16 weeks in <15 *Not Detected.
Finished 24 weeks treatment 3-17-18
SVR5 <15 Not Detected.
SVR 20 <15 Not Detected.
SVR 44 <15 Not Detected.Thank you Jesus.
Thank you Dr. James13 August 2017 at 12:26 am #26731Hello Betterdays,
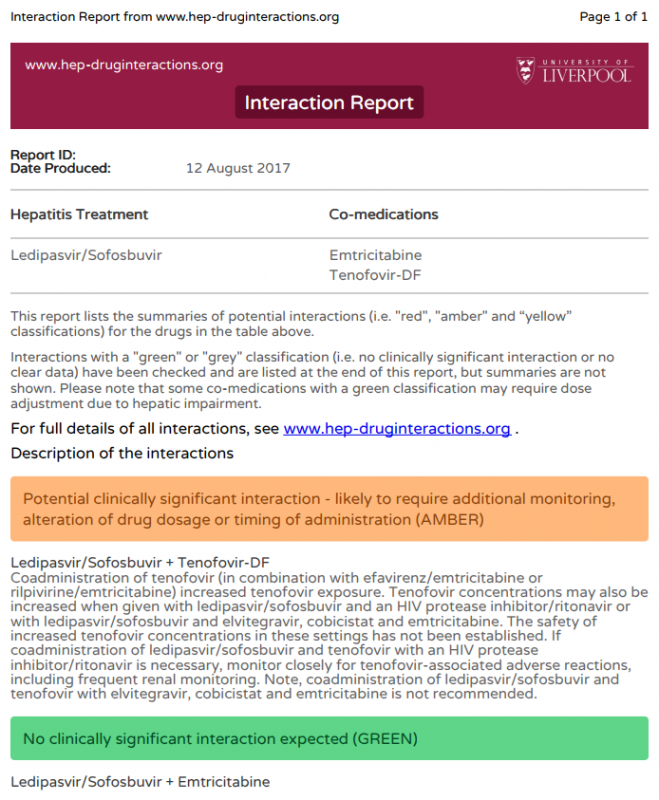
Following on from Splidog’s earlier reply I want to say, in case you have not already done this; to run your proposed DAA prescription, and PREP, or any other medications including herbals, through the Liverpool University Interactions checker.http://www.hep-druginteractions.org/checker
With very best wishes for your treatment, Mnem*(*
G2, infected maybe in 1971?
Diagnosed HVnon-A non-B 1980s, revised to HVC 1990’s.
Treatment naive. Fibroscan & bloods all normal ranges.
Viral load 7million,
began Redemption trial4, 12-week generic Sof/Vel (Incepta) 2017. Week 4 UND, Week 12UND, SVR24
Thank-yous to my doctor for the script, to Jan at FixHepC for wrangling, and to Dr Freeman for courage.
Kia kaha e hoa ma!13 August 2017 at 3:55 am #26733If you go to the interactions checker:
http://www.hep-druginteractions.org/checker
You can put in Ledipasvir/Sofosbuvir OR Daclatasvir/Sofosbuvir and then add in
Tenofovir and Emtricitabine (which is what’s in Truvada PrEP)
There is an interaction between PrEP and Harvoni but not between PrEP and Sovaldi/Daklinza so given either should work picking Sofosbuvir/Daclatasvir is sensible.
Velpatasvir also interacts in the same way as ledipasvir, so that’s not a good option either.
YMMV
13 August 2017 at 4:24 am #26734Welcome betterdays

I don’t know enough about Prep to provide any advice there. I would recommend an email to help@fixhepc.com – their team will be able to provide the appropriate and up to date medical advice regarding this.
You haven’t mentioned your genotype (GT1?) but the results you pulled up have the same ‘aggregate’ total of 96.8% so it looks like in your case the two treatments are comparable. Obviously they can’t both be listed first so I suspect the program has just listed one then the other. While the numbers from clinical trials for both are relatively limited in statistical numbers, they have been proved to be fairly accurate when compared with treatment results since then across the general population.
Regarding Harvoni, Sovaldi and Daklinza, these are the trade names of the originator manufacturers for their ‘branded’ products. Generics products while basically chemically the same have different trade names for their products although many patients still call these by the original manufacturer names. If you look at the “Notes” section below each recommendation you will see the various generics trade names.
More specific to your question, Sovaldi (sofosbuvir) was the big breakthrough drug that is the base for many treatments but requires other drugs added to create an effective combination depending on genotype, tx experience, etc.
Harvoni is made by the same manufacturer as Sovaldi. They combine the Sovaldi with one of their other drugs ( Ledipasvir) in one pill. Daklinza is made by a competitor of theirs so a Sofosbuvir/Daclatasvir combination is not available as a one pill original brand treatment. So commercial reasons rather than medical reasons.A quick summary of these three.
Sovaldi = Sofosbuvir (needs another drug such as Ledipasvir or Daclatasvir added so is a two pill combo)
Harvoni = Sofosbuvir+Ledipasvir (all in one pill)
Daklinza = Daclatasvir (needs sofosbuvir added so is a two pill combo)Best wishes and I’m sure those better days will soon arrive for you.

Edit: I see Dr Freeman has already responded with answers. I’ll leave this here as it may be of use to others wondering about the various names.
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND


 13 August 2017 at 11:39 am #26738
13 August 2017 at 11:39 am #26738Many Thanks Dr Freeman and others.
This site is invaluable, I would have never known about the interactions between the medications or the Hep C Checker from University
13 August 2017 at 11:57 am #26739So intent was I to tell you about the Liverpool University interactions checker, that I forgot to say “Welcome betterdays!”
I have gathered that you have Genotype 1 HCV from you being on this particular discussion thread.
It is great to have a PRep-er on this site, asking the questions that you asked. The more questions and responses posted here, about different situations that each of us is in, then the richer the information that is accessible for all.
Looking forward to hearing more from you on this journey.
Mnem *(*
G2, infected maybe in 1971?
Diagnosed HVnon-A non-B 1980s, revised to HVC 1990’s.
Treatment naive. Fibroscan & bloods all normal ranges.
Viral load 7million,
began Redemption trial4, 12-week generic Sof/Vel (Incepta) 2017. Week 4 UND, Week 12UND, SVR24
Thank-yous to my doctor for the script, to Jan at FixHepC for wrangling, and to Dr Freeman for courage.
Kia kaha e hoa ma!14 September 2017 at 4:34 am #26839Hi johnapple07,
Please be aware that while Sofosbuvir 400mg (Sovaldi) is a breakthrough drug, it is not a very effective HCV treatment by itself.
Treatment with Sovaldi requires its use in a combination treatment that includes at least one other drug such as Daclatasvir or Ledipasvir, either of which significantly increase treatment effectiveness.
Betterdays also needs to take other medications so it was suggested that based on those other medication’s potential for interactions, Sovaldi/Daclatasvir would be a more suitable treatment option for their situation than Harvoni.
G3a since ’78 – Dx ’12 – F4 (2xHCC)
24wk Tx – PEG/Riba/Dac 2013 relapsed
24wk Tx – Generic Sof/Dac/Riba 2015/16 relapsed
16wk Tx – 12/01/17 -> 03/05/17 NS3/NS5a + Generic Sof
SVR7 – 22/06/17 UND
SRV12 – 27/07/17 UND
SVR24 – 26/10/17 UND



-
AuthorPosts
- You must be logged in to reply to this topic.
