Forum Replies Created
-
AuthorPosts
-
Dear Coral,
Thank you very much.It made me cry. My mom cried too. There is a deeply satisfying feeling being part of this journey and having both the ability and trust to serve. I am not sure if something like this has happened before in realm of medicine where people from all parts of the world have selflessly united to support each other in treatment. I personally believe if everything can be and is rapidly getting globalized then why not healthcare? I think this is the first wave of globalization of healthcare which we are experiencing and before the end of this decade healthcare across borders will radically transform to where citizens have much more control on their health decisions than what Govt. is able to impose.
Thanks again for your lovely encouraging line.
Warm Regards,
Tim
MonkMed Care Team
http://www.monkmed.com
https://www.facebook.com/monkmedcareLast Christmas MonkMed completed 500 days. We received some really heartfelt messages where our users have poured open their hearts in their writings.A lot of them made us cry,All of them made us proud. We cannot be more thankful to them and God for considering us worthy enough to serve. We have made this ‘Spirit of MonkMed’ video compiling few of the hundreds we received. Am sure you will like it.
Congratulations Fara from entire team at MonkMed!
Hello Sheriff,
Many Canadians have benefitted by getting access to the medications from the FixHepC REDEMPTION trial.
Please email Help@FixHepC or Care@MonkMed.com with your phone number and we can call and walk you through the process of joining the REDEMPTION trials.
Thanks
MonkMed Care TeamCongratulations on getting UNDETECTED. Best Wishes for a youthful and healthy life from entire MonkMed Team!
UPDATE: July 27th 2016
As per the current timelines GILEAD Licensed Velpatasvir will be available in early September 2016 in India. Epclusa( GILEAD Velpatasvir) is already licensed to Gilead’s 11 Indian manufacturing partners which includes top brands like Mylan,Cipla & Natco which have undergone a complete transfer of technology(TOT). https://en.wikipedia.org/wiki/Technology_transfer.
Licensing does not mean just a business aspect of royalty sharing but more importantly ensuring the quality of the licensed product is exactly same as that of the original pill. This aspect is ensured by rigorous TOT which is explained below.
A complete technology of transfer(TOT) means the process of manufacturing of the pills is exact same as of the original GILEAD pill not just limited to the API but aspects of Excipient which is the NON API materials that goes into the manufacturing https://en.wikipedia.org/wiki/Excipient.
On top of the above, premium companies like Cipla and Mylan have their own Quality assurance processes. So even if pills are manufactured by Hetero and Hetero has its own Quality Assurance and Testing of the pills, after they hand over the pills Cipla and Mylan have their own comprehensive Quality Assurance and Testing process . We like this aspect of double testing which is the precise reason doctors world over trust Cipla and Mylan.
Mylan has grown from the third-largest generic and pharmaceuticals company in the United States to the second-largest generic and specialty pharmaceuticals company in the world.https://en.wikipedia.org/wiki/Mylan.
On top of all the above all Indian manufacturing units that manufacture or sell Licensed products are US FDA certified and have to keep up with rigorous standards also called Current Good Manufacturing Practices (CGMPs)
http://www.fda.gov/Drugs/DevelopmentApprovalProcess/Manufacturing/ucm169105.htm
Currently DCGI (Drug Controller General of India) approval for generic Velpatasvir is in process as the 11 Indian companies are gearing up production for early September launch.
30 June 2016 at 7:41 pm in reply to: Velpatasvir is not suitable for home compounding from API #20194One of the leading generic manufactures in India has an internal meeting July 17th on Velpatasvir. This will go through similar process as Sof and Sof+Led
1. Extensive licensing with patenting company(already done). Estimated number of companies to sell the licensed product is 11
2. Drug Controller General of India(DCGI) approval.Current estimates are its going to take longer than 1 month.
We will know more post the July 17th meeting.
Dear Meg,
Here are the lyrics and meaning. Its a classic song from Southern part of India and the team put a nice international touch to it.
Chanda mama raavayya : Moon please come
Nannu yetthukoni muddhuladi povayya : Hold me and kiss me and go
Maraalu nenenni chesina : Thou I always sulked and made petty demands
Gaaralyu neeve chupina: You always returned your TLC (tender loving care) and pampered meCheers
Tim
MonkMed Care Team
https://www.facebook.com/monkmedcare/Dear Meg,
You are most welcome and many thanks for clarifying.
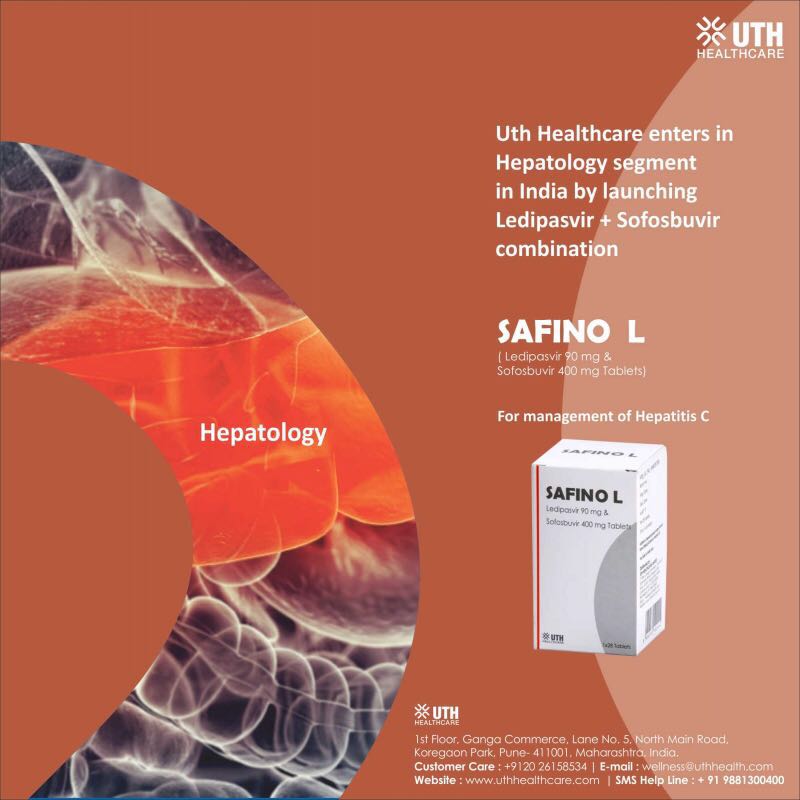
You are absolutely right. We also just heard back from UTH and they very recently launched into the Hepatology segment and the product is with very few stockists.
Attached is the flyer so that people can match the box if they are getting Safino L.
Below is the link from UTH .
http://uthhealthcare.com/hepatology/
Warm Regards,
Tim
MonkMed Care Team
http://www.monkmed.com
Tweets by MonkMedCare
https://www.facebook.com/monkmedcareAttachments:Dear Meg,
Congratulations! Great news and all the best for treatment completion!.
Needed a favor, could you please double check Safino L bottles says ‘manufactured’ from Hetero? And also if its ‘marketed’ by some other company? That will greatly help everyone in the forum and also us . We get a lot of questions around brand names and product packaging descriptons. Thanks for your kind help in this regard.
We work very closely with Hetero and have not heard of Safino L. Also double checked with Area Sales Manager of Hetero and they are not aware. Its late evening in India and we will call Sales Head tomorrow to be 100% sure. Also UTH which goes by name Emcure does not have Hep C medications as far as we know and their website
http://www.emcure.co.in/products_Pharma.asp
Its possible they have launched it recently we would like to know about it so that we can keep people informed.
For everyone’s general information, Hetero Sof+Led brand when launched was called LediSOF in some markets and LediFOS in some other. That caused a lot of confusion and concern about fake drugs. Also they changed the packaging in 3 months of launch adding to more confusion. In the end Hetero sent out a public apology and corrected the name to LediFOS. Its the name that was approved by DCGI(Drug Controller General of India). Here is the link.
http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=624686
Dear Zara,
It can be ordered through REDEMPTION Trials. Here is a recent case that went through full Health Canada approval.Fedex is not the authority here.
Please read willeert’s post
http://fixhepc.com/forum/daa-access/385-monkmed.html?start=45#15221The process to sign up for REDEMPTION trials is here
http://fixhepc.com/redemption-imagine-freedom-from-hepatitis-c
You can mail help@FixHepC.com for further information.
FixHepC guarantees delivery and comes with doctor advise for duration for treatment.
Regards
Tim
MonkMed Care Team
http://www.monkmed.com/
Tweets by MonkMedCare
https://www.facebook.com/monkmedcareHere is link to the details on price reduction. Also lists names of all other drugs
We have been assured not more than 4 days delay. So doesn’t look too bad.
Dear Mike,
No we did not travel. Maybe next year. Dr Debasis should have travelled but he is very busy with REDEMPTION consultations and rest of us are busy ensuring shippings go through. We are continuously on phone trying to clear packages. Due to heightened security customs clearances worldwide is taking longer than usual.
Warm Regards
Tim
MonkMed Care TeamCongratulations PauliNO, Great news!!!
Cheers,
Tim,
MonkMed Care TeamMonkMed works with GILEAD licensed medications only. For REDEMPTION-3( Sof+ Led) it is Cipla Hepcvir L and for REDEMPTION-2 it is Mylan MyHep(Sof) and MyDacla(Dac). Because the medications are from reputed pharmaceutical companies( E.g. Cipla is big name in UK and Mylan is a US manufacturer) doctors of respective countries have been more forthcoming to monitor patients as they are more comfortable with brands they prescribe for other ailments.
All REDEMPTION participants avail MonkMed doctor consultation services at no additional cost for duration of treatment of 12-24 weeks and if required any post treatment.
This link has some of our esteemed participant’s kind words about us.
https://fixhepc.com/component/search/?searchword=monkmed&searchphrase=all&Itemid=271
-
AuthorPosts