Super User
EASL 2017 Generics Results
Here is a copy of the generic hep c medication presentation given by Dr James Freeman at EASL 2017 today.
Mostly the presentation followed the slides.
The last words, with the last slide were "I want everyone to join the club, not the hep c buyers club, this club, the hep C cure club."
Click here to get the full slide set on generics presented at EASL 2017 in Amsterdam
A Brief History Of Sofosbuvir
“The Liver Meeting”, or AASLD 2011, was taking place at the great, glass-fronted Moscone West Convention Center in downtown San Francisco. Almost 9,000 people were there – family doctors, hepatologists, gastroenterologists, academics, drug salesmen, public health officials – all networking, trudging the escalators, wandering the beige exhibit halls, trying to stay on top of an avalanche of medical information. Across five days in early November, no fewer than 2,200 liver-related scientific abstracts were being presented, at sessions that ran from 6.30am until 9pm. Specialists in hepatitis C, an awkward, under-recognised health problem, were used to finding their way to the back rooms, the less-glamorous receptions.
Many felt it was time the disease moved up the agenda. Since it was identified in 1989, “Hep C” or “HCV” to those who work on it, has only confounded those who underestimate it. Transmitted through blood, the virus is now thought to infect around 2.5 per cent of the world’s population, or 170 million people. Of those, around a quarter will develop liver cirrhosis, or cancer, within a decade or two. In the UK, deaths from hepatitis C have trebled since 1996 and more than 200,000 people are infected. No one knows what to do about the coming demand for liver transplants. For the past decade at least, scientists have used phrases such as “gathering storm” and “silent epidemic” to try to bring attention to the subject.
There has also been a torrent of new data to keep up with. Despite its orphan status, the sheer numbers of people infected with hepatitis C have made it the subject of hundreds of millions of dollars of medical research – particularly by pharmaceutical companies, working on a new generation of drugs. And AASLD, as the world’s premier liver event, has become the occasion when information on those drugs – known as “direct-acting antivirals” – is often shared for the first time. In recent years, hepatitis C delegates had got used to turning up to must-see presentations that were filled way beyond capacity.
One look at the 2011 programme told conference-goers that a session on the Sunday afternoon – “HCV: Refining the Use of Direct-Acting Antivirals” – was going to be mobbed. Room 2001, on the second floor of the convention centre, only held about 400 people. Dr Ed Gane, a 51-year-old hepatologist and transplant specialist from New Zealand, who was to give the fourth of six presentations in the session, arrived about 10 minutes before it was due to begin. “It was packed to the gunwales,” he told me. Conference staff began setting up a video link to adjoining rooms to cope with the spillover.
The cognoscenti were there to hear Gane. An unshowy, respected doctor, he had been working on hepatitis C since the early 1990s, and for the past year, had been carrying out a clinical trial of a particularly promising new drug developed by a small American pharmaceutical company called Pharmasset. The drug was known by its laboratory name, “PSI-7977”, and today, Gane was to give an update on the trial, called ELECTRON.
The Story Of Generics Hits The Lancet
An article with the title: Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries just made it into the Lancet print edition:
http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)32051-7/fulltext
There is a PDF of it here
Debates in Hepatology - The use of generic medications for hepatitis C - The Case For

This article about the rationale for using generic hepatitis C medication has just been published in Liver International in their section "Debates in Hepatology".
James A. D. Freeman1 and Andrew Hill2
1 GP2U Telehealth, Hobart, Tas., Australia
2 St Stephens AIDS Centre, Chelsea and Westminster Hospital, London, UK
Liver Int. 2016; 36: 929–932. DOI: 10.1111/liv.13157
Abstract
Hepatitis C, hepatitis B, HIV, TB and malaria are the five major causes of infectious disease death worldwide. In a breakthrough that rivals the invention of penicillin, drugs that cure hepatitis C, with minimal side effects and high success rates, have reached the market, but, in what must be one of the greatest tragedies of modern times, these life-saving medications are not being deployed on a mass scale. Pharmaceutical patents are gifted to private corporations by governments for the dual purposes of protecting R&D expenditure and encouraging innovation. Unfortunately the monopoly pricing power these patents provision currently lacks adequate checks and balances, is open to abuse, and is quite clearly being abused. The sort of legislative changes required to deliver on the original goals of pharmaceutical patents will take years or even decades to eventuate. Parallel importation of generic medication offers hope to the millions of patients with HCV unable to afford access to vastly overpriced originator medications. Doctors prescribing and monitoring patients taking generics can take comfort from the fact that the REDEMPTION trial results show, like the HIV generics that came before them, that HCV generics deliver robust clinical results.
Read the full article at: http://onlinelibrary.wiley.com/doi/10.1111/liv.13157/full
To find out more about generic hepatitis C medication please visit our forum where you can read the stories of patient successfully accessing and treating their hepatitis C with affordable generic medication.
New Russian Forum for HCV - With Doctors - http://gepatitka.ru/
Мы рады представить некоммерческий проект «ГЕПАТИТКА», созданный группой активистов для поддержки, помощи и общения людей, затронутых вирусными гепатитами B, C и D.
Начало этого проекта было положено в марте 2012 года пациентом из Москвы во время того, как он проходил курс лечения гепатита С пегилированнымиинтерферонами и рибавирином. Чтобы облегчить проживание сложных побочных эффектов, связанных с лечением, молодой человек начал взаимодействовать с людьми, затронутыми гепатитом С. Он делился своим опытом, рассказывал, как передается гепатит С, мотивировал лечиться, а не ждать развития фиброза, пояснял, как можно поддержать человека на лечении, организовывал совместное времяпровождение и он-лайн взаимодействие людей, связанных с гепатитом С. Вокруг него образовывалось сообщество людей, связанных с гепатитом С. Сообщество разрасталось, вышло за пределы Москвы и в октябре 2013 года его товарищ из Минска, замотивированный на лечение гепатита С, создал группу в социальной сети фейсбук. Этой группе далось запоминающееся название – ГЕПАТИТКА.
В 2015 году проект переформатируется, благодаря присоединению к работе и разносторонней поддержке коллег из ITPCru. Весной 2015 года в группе ГЕПАТИТКА в фейсбуке начала появляться информация о прорыве в лечении хронического гепатита С в виде появления на рынке препаратов для лечения гепатита С с принципиально новым принципом действия, с почти 100% эффективностью, легких по переносимости и с сокращенным сроком лечения. ГЕПАТИТКА была одним из первых ресурсов, где появились новости по доступу к лечению ВГС в мире новыми безинтерфероновыми схемами, где были опубликованы перечни доступных безинтерфероновых генерических препаратов для лечения ВГС с указанием цен и производителей. Ресурс подкреплял новостную информацию о препаратах переводенными на русский язык международными клиническими рекомендациями по лечению новыми схемами, а также результаты КИ по ним. При этом составом координатором ГЕПАТИТКИ было принято решение поддерживать исключительно традиционные мировые методы диагностики и лечения. Благодаря выкладываемой информации, постоянной работе с пациенстким сообществом и опыту первопроходцев, кто уже не мог откладывать лечение и воспользовался новыми препаратами, появилось много информации о ходе лечения новыми препаратами в режиме он-лайн, и как следствие, много возможностей расширения доступа к лечению гепатита С для всех нуждающихся и очевидная необходимость развития проекта ГЕПАТИТКА в разных направлениях.
На июнь 2016 года проект представлен в следующих ресурсах:
- закрытая группа фейсбук (3477 участников): https://www.facebook.com/groups/NAHCV/
- закрытая группа в ВКОНТАКТЕ (475 участников): https://vk.com/gepatitkaru
- сайт http://gepatitka.ru/, где доступен пациентский форум, а также последние мировые рекомендации по лечению ВГС и бесплатное медицинское консультирование по вопросам хронических гепатитов в режиме он-лайн.
На сегодня проект ГЕПАТИТКА координируется небольшой группой активистов из Москвы, Санкт-Петербурга и Минска. В планах стоит расширение развития в регионах ВЕЦА, в проекте на ежедневной основе задействованы активисты из РФ, Украины и Латвии. В проект вовлечены два медицинских специалиста, практикующих гепатолога, из Германии и России.
Добро пожаловать всем, кто затронут вопросами гепатитов на площадки проекта ГЕПАТИТКА. Вы сможете найти много полезной информации и общения там.
------
Dear all, especially Russian speaking audience,
We are pleased to present a non-profit project "GEPATITKA" for the Russian audience. Project was created by a group of activists to support and to coordinate communication between people, affected by viral hepatitis B, C and D.
Project started in March 2012 while a patient from Moscow (Russian Federation) was undergoing pegylated interferon and ribavirin treatment for hepatitis C. In order to handle tough side effects and not to stay alone with his condition, he started to share his experience with others, started to learn more about HCV and to share his knowledge. Very soon a community of patients grew around him. He treated his HCV and continued his activities to help others. He motivated his friend from Belarus to start treatment as well and in October 2013 the project appeared on-line, on FACEBOOK, and got it name – GEPATITKA (Gepatit means Hepatitis in Russian).
In 2015 the project GEPATITKA was reformatted (got a new structure), due to the joining of ITPCru. GEPATITKA started to spread out information about new landscapes in HCV treatment, about the DAA appearance in the world with almost 100% efficiency, easy portability and a reduced period of treatment. GEPATITKA was one of the first resources in the EECA region, where information about PEG-IFN-free regimens was published and lists with DAA generics with prices and producers appeared. Project supports its publications with the international clinical guidelines, and only traditional methods of HCV diagnosis and treatment. Due to the strong informational work and very attentive and intense approach to the patient’s community project GEPATITKA became very popular in the Russian speaking countries, especially in Russia, Belarus and Ukraine.
In June 2016 GEPATITKA project is presented in the following resources (only in Russian language):
- Private group on Facebook (more than 3500 participants): https://www.facebook.com/groups/NAHCV/
- Private group in a Russian social network VKontakte (around 500 participants): https://vk.com/gepatitkaru
- at the web site GEPATITKA: http://gepatitka.ru/
This web site provides not only the latest world updates for HCV treatment and free medical consultations for chronic hepatitis in the online mode, but also a platform for the patient’s forum.
Today GEPATITKA project is being coordinated by a small group of activists from Moscow, Saint Petersburg and Minsk (Belarus). They plan to expand in the EECA regions. A number of volunteers, who are affected by hepatitis, from Russia, Ukraine and Latvia are involved in this project on a daily basis. Two medical specialists, hepatologists from Germany and Russia answer questions on the web site free of charge on the daily basis.
Resources of GEPATITKA are very friendly and patient - oriented and warmly welcomes everyone who is affected by hepatits.
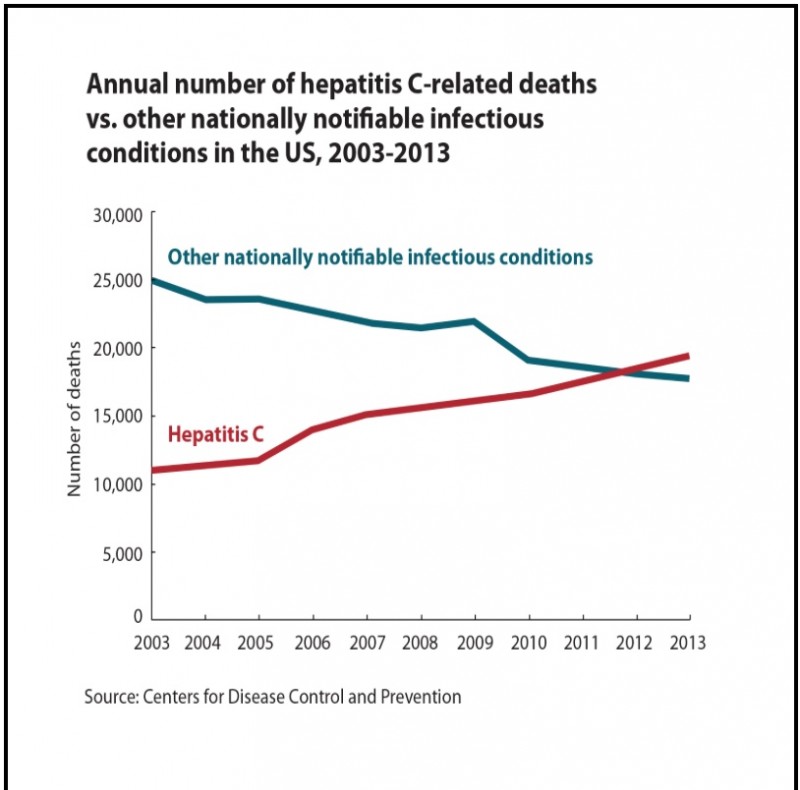
CDC - Hepatitis C death rate has overtaken all other notifiable infectious condition in USA
Hardev Karar we salute you. R.E.S.P.E.C.T
What Happened to the Indian Official that Rejected the US Drug Company Gilead’s Patent Application in 2015?
It appears that being a man of honour is less valued than it should be.
http://www.caravanmagazine.in/vantage/indian-official-rejected-gilead-patent-forced-out
Hardev Karar, I salute you as a man of honour and integrity.
Dr James Freeman
One poignant post that says it all...
Gilead is a hedge fund under the guise of a pharmaceutical company. They want drugs that will treat diseases that are problems in wealthy, industrialized nations. Don't look for them to come up with a cure for malaria, ebola or Zika-there is just not enough money in it for them to be interested.
They will continue to tend the geese that lay the golden eggs until, like Australia, they are forced to "make the deal". Then they will cook and eat them.
In the US, insurers are relaxing restrictions because of legal pressure brought about by patients over denial of treatment. This will force substantial rises in the cost of health insurance for everyone, with Gilead getting the money. Health insurance companies don‘t go broke. They have reserve funds, and when they have to tap into them one year, they raise the premiums the next to cover the shortfall.
Widespread acceptance of affordable generics is the only way Greediad will have its hand forced. Dr. Freeman's EASL presentation on the stellar findings of the REDEMPTION clinical trials tomorrow could be a major first step.
We are in a high stakes poker game here. The insurance companies are the "house”- Gilead, the well-funded, card-counting professional gambler.
We are the chips.
PBS Pricing - Take a deep breath....
So I guess like a lot of people I've been wondering how good a deal the PBS negotiated.
Last week I heard that a major public hospital had ordered 10 lots for $66,000 but thought that must be wrong.
Given the pharmacists have to order these medications I came up with the cunning plan of asking one. The prices were not available earlier in the week, but they are now.
Make of it what you will but the $22 k per month price for Harvoni looks like exactly the same as the price France and Germany negotiated and would allow 15,000 people to be treated for $1 billion.
G'day,
Here is a list of the three medications and the quotes from our suppliers. I did some further investigation after our phone call and found that the prices on the websites were completely wrong. I called the suppliers and got the correct quote. As such, I have not attached any screen shots - as these would be very misleading!
- Daklinza 60mg 28 tablets- $7,736.30
- Harvoni 90/400mg 28 tablets- $22,135.00
- Sovaldi 400mg 28 tablets- $19,367.00
Stock for Harvoni and Sovaldi are not available with our suppliers yet, but they have ordered it - and so hopefully it isn't too far away (but they could not give me an ETA).
If you have any further questions please do not hesitate to ask either myself or J.
Kind Regards,
D
Here is why the VA (US Government) owns sofosbuvir
PSI-6130 www.ncbi.nlm.nih.gov/pmc/articles/PMC1932527/
ACKNOWLEDGMENTS
This work was supported in part by NIH grants 5R37-AI-041980, 4R37-AI-025899, 5P30-AI-50409 (CFAR), and RR000165 (to Harold M. McClure) and the Department of Veterans Affairs.
R. F. Schinazi is the principal founder and a former director of Pharmasset, Inc. He is also a major shareholder of Pharmasset, Inc., and Idenix Pharmaceuticals.
Now PSI-7851 comes directly from the development of PSI-6130
www.wikinvest.com/stock/Pharmasset_(VRUS)/Psi-7851
PSI-7977 (aka Sofosbuvir) is the active metabolite of PSI-7851 so is also directly related to PSI-6130...
files.shareholder.com/downloads/VRUS/0x0...4533817aa73/PSI-7977
So the baby (invention) was born from NIH and VA grant money and was created by someone who was 7/8 time employed by the US Government.
So what you say?
Executive Order 10096, issued by President Harry S. Truman in 1950, I say.
www.tms.org/pubs/journals/JOM/matters/matters-9004.html
The main section of the executive order provides that the government shall obtain all rights to any invention made by an employee if any one of the following conditions applies: the invention is made during working hours; the invention is made using either government facilities, equipment, etc., or is made with the help of another government employee who is on official duty; or the invention relates to the official duties of the inventor.
So what did Dr Schinazi do in 2007-2010 and what were his "official duties"?
This document indicates he was employed by the VA as a research chemist and did indeed make the invention in relation to "the official duties of the inventor" as in on government time, in a government lab.....
tel.archives-ouvertes.fr/pastel-00605911/document
The research presented in this dissertation was performed in the Laboratory of Biochemical Pharmacology (LOBP) at the Veterans Affairs Medical Center/Emory University. LOBP is a drug discovery and preclinical drug development laboratory directed by Dr. Raymond F. Schinazi.
Developed in the 1/8 non government time. This is fantasy, fiction, and BS.
Developed on the government ticket. Owned by the US government.
Brilliant, breakthrough and stellar but not done independently.
And it is all documented.