Wednesday, 19 September 2018 19:43
Cabozantinib delivers hope for patients with HCC failing Sorafanib
Written by Super User
For patients with Hepatocellular Carcinoma (HCC) the options are limited. Caught early enough RadioFrequency Ablation (RFC), TransArterial ChemoEmbolisation (TACE) or surgical resection can potentially be curative. For some patients with advanced disease a liver transplant may be an option, but what about everyone…
Strictly speaking, this relates to HIV but it also applies the Hepatitis C.Undetectable = UntransmissableGet tested, get treated, get cured! https://ashm.org.au/HIV/UequalsU/
Thursday, 26 July 2018 12:12
Great News For Australian Patients - MAVIRET® (glecaprevir/pibrentasvir) PBS listed on 1 August 2018
Written by Super User
News FlashMAVIRET® (glecaprevir/pibrentasvir) was listed on the PBS in Australia today. AbbVie is pleased to announce that MAVIRET is to be listed on the PBS on 1 August 2018 for the treatment of chronic hepatitis C virus (HCV) infection in adults. MAVIRET…
Tagged under
Most of the messaging to people about Hepatitis C is boring with as much cut through as a blunt knife through a block of titanium. If you were born between A and B, if this, if that, etc. It's time to change…
The other day, on Facebook, I witnessed a patient, looking for advice, be given some very poor advice. Where poor = wrong and potentially lethal. I provided some accurate commentary which was deleted, leaving a whole lot of no doubt well-meaning, but…
Sunday, 08 April 2018 08:41
Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals
Written by Super User
They say (who are they anyway?) "If it seems to good to be true it probably is". When it comes to generic Hepatitis C medication some people struggle with the idea that something like Harvoni® with a list price of $94,000 USD…
Tagged under
Tuesday, 13 March 2018 12:20
Management Of Patients With HCV Who Have Achieved SVR
Written by Super User
So you've made it! SVR at last. What now? Here is a slideset from Clinical Care Options where Ira M. Jacobson, MD, and Paul Y. Kwo, MD, review optimal management of patients with HCV who have achieved SVR, including recommendations for HCV RNA and HCC monitoring. These doctors are…
Friday, 09 March 2018 15:07
Perfectovir - Abbvie Glecaprevir + Pibrentasvir (Maviret) + Sofosbuvir Shines
Written by Super User
With HIV we observered that if we use 1 drug resistance develops rapidly, with 2 drugs it is slower, and with 3 drugs it virtually never happens, so it does not come as any great surprise that combining an NS3/4A drug with…
Saturday, 13 January 2018 09:47
Effectiveness of ravidasvir plus sofosbuvir in chronic hepatitis C genotype-4
Written by Super User
Highlights Ravidasvir is a new NS5A inhibitor for HCV. Sofosbuvir + Ravidasvir with or without RBV has achieved very high SVR rates. Results are comparable for both patients with and without cirrhosis. Serious adverse events were noticed in very few treated patients.…
Monday, 01 January 2018 19:19
How to take the new HCV DAA Medications like Epclusa and Vosevii
Written by Super User
A quick note on how to take your DAA medication. Read the package insert and follow the instructions with respect to with (or without) food. For example, for Vosevii Administration with food enhances the oral bioavailability of sofosbuvir, velpatasvir, and voxilaprevir. Relative…
Any SVR rate <100% means a small number of people will not attain SVR. A 95% SVR means for every 20 people on generic treatment 1 patient will relapse. Back in early 2016 I came across an excellent article from Dr Jordan Feldwas…
Once upon a time, there was an old man who used to go to the ocean to do his writing. He had a habit of walking on the beach every morning before he began his work. Early one morning, he was walking…
Friday, 01 December 2017 09:17
Sofosbuvir+Daclatasvir results shine - bad news for Epclusa and Maviret?
Written by Super User
The combination of Sovaldi (Sofosbuvir) and Daklinza (Daclatasvir) was the world's first pan-genotypic Hepatitis C treatment. In the USA and Europe the $140,000 price tag of this combination means it has seen relatively little use, but in the world of generics it…
Tuesday, 21 November 2017 08:27
What happens when you finish your Hep C treatment?
Written by Super User
Recently I have been getting a number of enquires from patients who have just finished their tablets, all asking the same questions, so here is a quick primer. If you are looking for this sort of information you will find a lot…
Friday, 17 November 2017 07:50
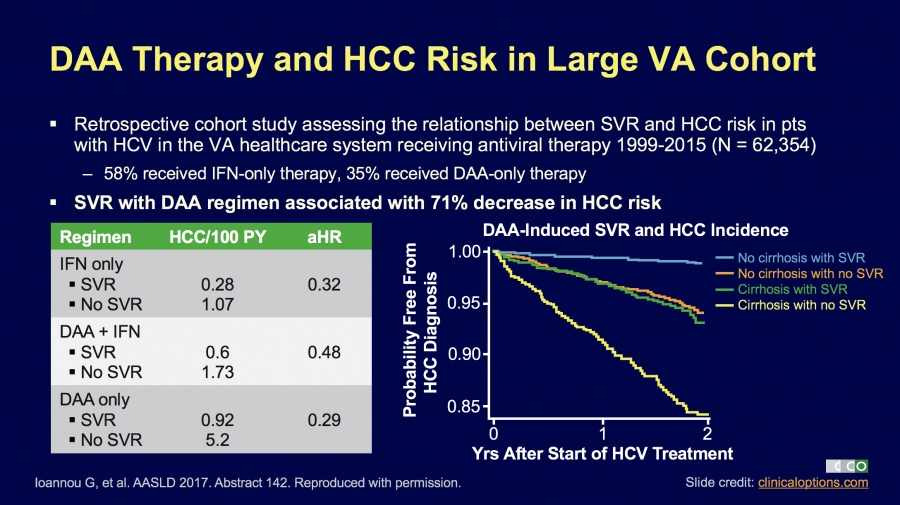
71% decrease in cancer rate for Hep C patients with SVR
Written by Super User
Some time ago we saw some very bad science published. This suggested that treating Hepatitis C confers no benefits. While experts around the world expressed their firm view this was complete and utter rubbish here is something simple to understand. Get treated,…
Monday, 13 November 2017 16:26
Pharmaniaga, Pharco and DNDi Sign Agreement to Provide Affordable Hepatitis C Treatment in Malaysia
Written by Super User
KUALA LUMPUR/GENEVA – 13th November 2017 – Malaysian pharmaceutical company Pharmaniaga Logistics Sdn Bhd (Pharmaniaga), Egyptian pharmaceutical company Pharco Pharmaceuticals (Pharco) and non-profit research and development organization Drugs for Neglected Diseases initiative (DNDi) have signed a collaboration agreement to supply a new…
Friday, 03 November 2017 22:37
We have the power to FixHepC, but the real question is...
Written by Super User
We have the power to FixHepC, but the real question is... Do we have the willpower? This was how Dr James Freeman ended his presentation at the World Hepatitis Summit in Brazil. You can watch the full presentation on YouTube. In essence,…
Tagged under
Wednesday, 01 November 2017 20:09
Qualifying for Social Security Disability Benefits With Hepatitis C
Written by Super User
Hepatitis C is a disease that can make you feel old before your time. While there is no doubt that treatment with the new generation of direct acting antivirals like Harvoni is by far the best option this is not available to…
Tagged under
Thursday, 19 October 2017 12:12
If you have Hepatitis C where can you get free confidential support?
Written by Super User
About 1 in 100 people, from all walks of life, have Hepatitis C so it is pretty common. Because it is a highly stigmatised disease, linked in the public mind to IV drug use, most patients choose to keep their diagnosis confidential.…
Thursday, 05 October 2017 12:08
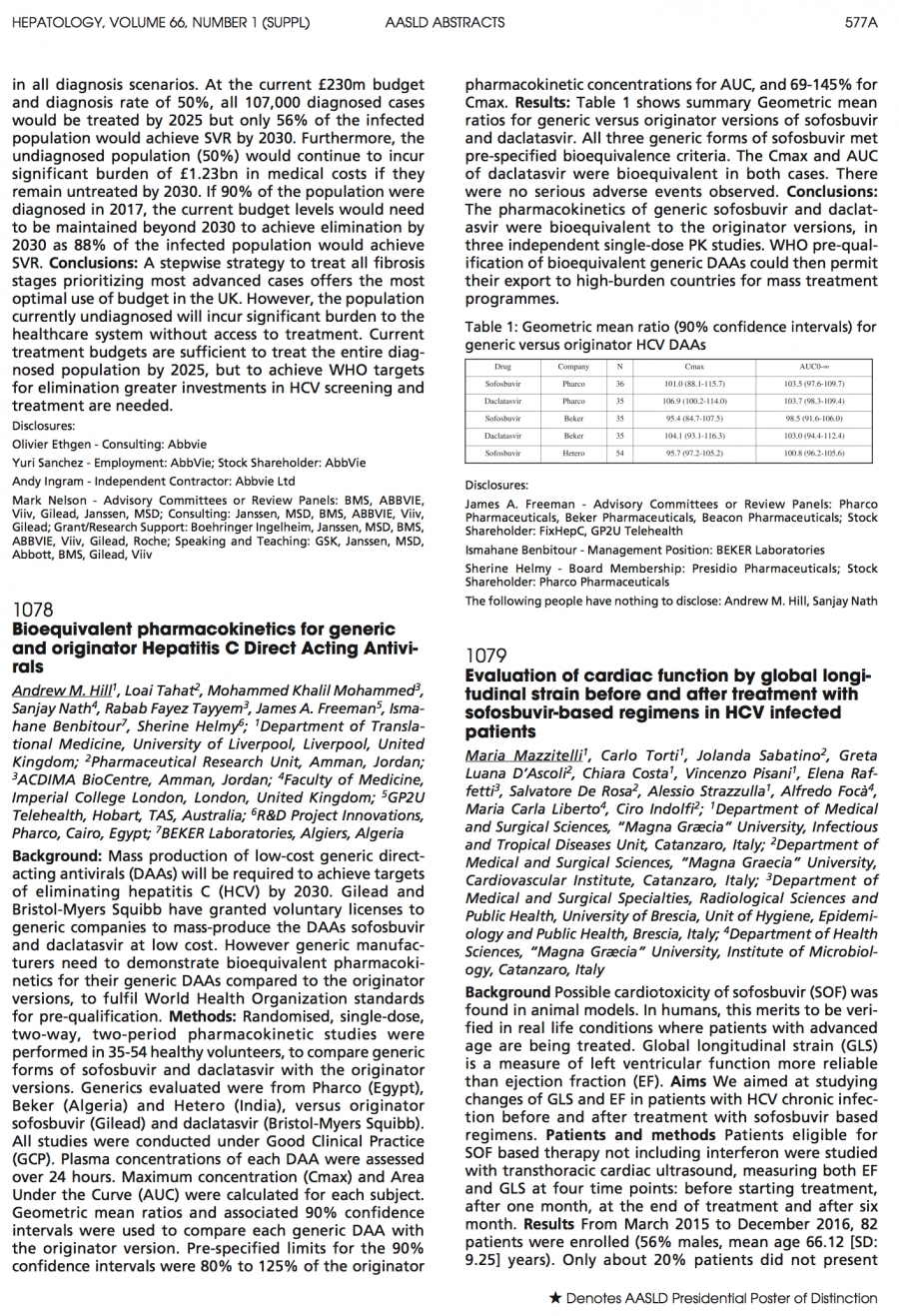
AASLD 2017: Generics shown to be bioequivalent to originator brands
Written by Super User
Just out of embargo for AASLD 2017 is the rather innocuous sounding Abstract 1078 which says, in brief, that these generic DAAs are inarguably proven the same as the originator DAAs.Bioequivalent pharmacokinetics for generic and originator Hepatitis C Direct Acting Antivirals Andrew M.…
Tagged under
Thursday, 21 September 2017 15:34
Bye bye limitatio (and generics) - Open access to the new Hepatitis C medications for all Swiss patients
Written by Super User
Switzerland has just announced that all limitations on access to the new DAAs will be abolished allowing treatment for all. Great news for Swiss patients. Here is an article from the Swiss Hepatitis C Association about it. If you are not lucky…
What exactly is chronic HCV? In a nutshell, it refers to ongoing inflammation of your liver. But it can lead to symptoms throughout your body. Over time, living with this condition can cause your body to be especially vulnerable to serious health…
Last week there was some celebrations after Under pressure, Gilead expands Sovaldi licensing deal to four middle-income countries. So great news, right? Not exactly, because in order for a country to have PRACTICAL ACCESS the product needs to be registered for sale…
Tagged under
Friday, 11 August 2017 11:11
AbbVie's New Hepatitis C Treatment Won't Cure Patient Access Issue
Written by Super User
By Priti Krishtel Last week, the FDA approved AbbVie’s Mavyret—a new hepatitis C virus (HCV) drug that treats all genotypes of the disease and cures more than 90% of patients within just 8 weeks of treatment. This has been reported as a…
More...
Friday, 11 August 2017 09:38
Breaking News: Study Shows HCV DAA Treatment Demonstrates 57% Survival Benefit
Written by Super User
"To our knowledge, this is the first large-scale study to demonstrate the effect of newer DAA regimens upon survival. Treatment with 2 commonly used DAA regimens, PrOD and LDV/SOF, was associated with significant improvements in survival within the first 18 months of…
Tagged under
Here is a copy of the presentation, called "Practivism, it's the new black", given by Dr James Freeman at the International AIDS society meeting in Paris today. Here is the text that went with the images. Crisis, What Crisis? This crisis. Look at this…
Over the past year there has been quite a lot of discussion about the risk of liver cancer following treatment with Direct Acting Antivirals like Harvoni® and Sovaldi®. Here is a quick explanation and the latest thinking from the academic gurus. First,…
Saturday, 08 July 2017 12:42
EASL Response to the Cochrane Systematic Review on DAA-Based Treatment of Chronic Hepatitis C
Written by Super User
Here is the response from EASL to the recent Cochrane review. http://www.journal-of-hepatology.eu/pb/assets/raw/Health%20Advance/journals/jhepat/CochraneEASLJMP003.pdf EASL, the European Association for the Study of the Liver, one of the world leading associations of liver specialists, feels compelled to express its serious concerns after the recent publication…